Fig. 11.1
Shown here is a linear tip echoendoscope. This type of tip creates a sagittal view

Fig. 11.2
Shown here is a radial tip echoendoscope with the balloon inflated. This type of tip creates axial views
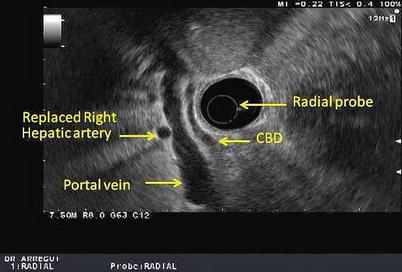
Fig. 11.3
This is a radial view of the porta hepatis obtained with a radial echoendoscope. CBD common bile duct
The linear echoendoscope creates a sonographic image that is parallel to the shaft of the scope resulting in a sagittal sector image as opposed to the circumferential cross-sectional image of the radial echoendoscope [3]. This allows the operator to trace the path of a needle as it is inserted out of the operating channel in real time and thus enables therapeutic capabilities and interventions such as EUS-guided FNA (fine needle aspiration biopsy), EUS-guided injection therapies, and EUS-guided drainage procedures [2]. The standard linear echoendoscope is actually curvilinear and oblique viewing, similar to the side-viewing duodenoscope used for ERCP. It also has a lever on the handle called the elevator that raises the instruments passed through the accessory channel and thus allows fine movements. This configuration carries some inherent drawbacks. First, linear and radial echoendoscopes only allow side-viewing endoscopy as opposed to the more intuitive forward viewing of a gastroscope, for example. There is also a “push back phenomenon,” where the force of the needle advancement might cause the scope to push back. Finally, the size of the accessory device is limited by the angulation of the accessory channel at the endoscope tip and the elevator [4]. Forward-viewing curvilinear echoendoscopes have been recently developed to overcome these challenges with the theoretical advantages of superior endoscopic visualization, easier deployment and manipulation of devices and needles, as well as better transmission of force to the needle. These forward-viewing scopes also have an increased tip deflection, but they also have a narrower ultrasound scanning range and lack an elevator. Early data on forward-viewing echoendoscopes suggest EUS visualization is comparable to the oblique-viewing linear echoendoscope in the upper gastrointestinal tract. Furthermore, endoscopists have reported increased ease of device deployment and better force transmission [4]. Currently, these forward-viewing echoendoscopes are mainly used for research purposes.
In addition to the conventional linear and radial echoendoscopes, catheter mini-ultrasound probes and intraductal ultrasound (IDUS) probes have been developed. The catheter miniprobes can be used for lesions in or near the gastrointestinal mucosa or when an obstruction precludes the safe use of an echoendoscope. Their outer diameter ranges from 1.7 to 3.1 mm, which allows their passage through the working channel of an upper endoscope or duodenoscope (Fig. 11.4). These probes have a limited life span due to breakdown of the driveshaft that spins the ultrasound transducer tip, especially when used through a duodenoscope because the elevator causes repeated trauma to the probe.


Fig. 11.4
A minicatheter can be seen through the working port of a duodenoscope
Transpapillary IDUS catheters (Fig. 11.5) are high-frequency wire-guided catheters that are typically used during endoscopic retrograde cholangiopancreatography (ERCP). These probes produce high-quality cross-sectional images of the pancreatic and biliary ducts with resolution ranging from 0.07 to 0.18 mm. Not only are these probes high frequency, but the fluid in the ducts where they are inserted serves as an excellent acoustic window which improves resolution [2]. The use of IDUS to evaluate the biliary tree was first published in 1992, but despite reports indicating its value in decreasing the rate of recurrent biliary stones after endoscopic sphincterotomy, its utilization in clinical practice remains low [5]. Other indications include cholangiocarcinoma, evaluation of pancreatic cystic tumors, pancreatic islet cell tumors, and biliary and pancreatic duct strictures.


Fig. 11.5
An intraductal ultrasound is passed on a guidewire catheter through the working port of a duodenoscope
Console Function
It is essential to be familiar with the various functions of the EUS console as well as to understand when to apply the different US functions when acquiring images in order to make more accurate clinical diagnosis. We will discuss some of the important functions. The depth/range function changes the display depth of the image. It is helpful to start with the greater depth range for initial scanning and identify any gross abnormality and magnify the near-field view for more detailed study. Similarly, using the frequency function, one should start with a lower frequency to scan through a wide range of structures. Indeed lower frequency allows greater penetration but lower resolution. Once a lesion is identified, frequency can be increased to obtain a better resolution, which refers to the ability to discriminate between two points along the beam path. The focus function allows convergence of the US beam to a particular depth to achieve an image with a higher lateral resolution. The gain function adjusts the overall sensitivity of the gray-scale image. If it is turned too high, the image will be too white, and if it is turned too low, the image will be too dark. The Doppler function not only allows identification of blood flow in vessels but also provides information regarding the direction of the flow and its velocity. The power Doppler function has a higher sensitivity in detecting blood flow because background noise is reduced. However, it does not give any information on flow direction and velocity. Annotations features are also available to measure and mark any structures or lesions [6]. (Refer to section “Control panel” in Chap. 3 for more information.)
General Technique of Use
Most echoendoscopes are oblique viewing and the process of pharyngeal intubation is nearly blind similar to a duodenoscopy. The echoendoscope is inserted into the pharynx with the tip deflected downward. Once in the pharynx, the tip is gently advanced in the esophagus. Forceful intubation must be avoided to prevent perforation. In difficult cases, excluding a Zenker’s diverticulum or other unusual anatomic abnormalities, a diagnostic gastroscopy may be advisable. For both radial and linear EUS, recognition of key landmarks is vital for proper orientation. Filling the GI lumen with water is helpful when the GI wall is being examined. Radial images are axial circumferential images that are more easily interpreted partly because axial imaging is more familiar to most. Linear images are sagittal sector images that are more limited and more difficult to interpret. To facilitate performance of the exam and interpretation of the image, linear EUS requires the use of key movements, which include advancement and withdrawal, clockwise and counterclockwise torquing, and angulation. Torquing is achieved either by using the right hand to torque the shaft of the echoendoscope or by changing the direction of the handle by turning the left wrist or body. Angulation of the tip is mainly performed by using the up-down control. It is also important to ensure proper coupling by continuous suction and by keeping the tip of the echoendoscope pressed against the mucosa. For example, with a linear scope in the mediastinum, the abdominal aorta should be identified first as a large hypoechoic tubular structure with the echoendoscope shaft held at neutral position. Doppler can confirm vascularity. As the scope is advanced distally in the esophagus into the proximal stomach, the celiac artery (Fig. 11.6) and superior mesenteric artery should be seen next arising from the aorta. At the level of the celiac artery, clockwise rotation allows examination of the left adrenal gland (Fig. 11.7) superior to the kidney, while counterclockwise rotation will allow visualization of the left lobe of the liver [3].
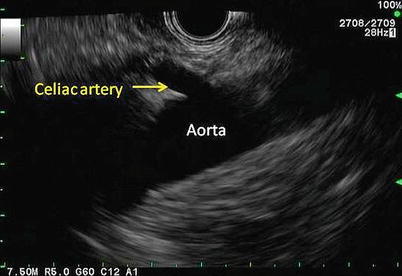
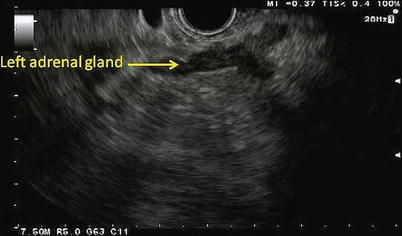

Fig. 11.6
The celiac artery can be seen coming off the aorta. This view is obtained through a linear echoendoscope

Fig. 11.7
Depicted here is the typical sonographic appearance of a left adrenal gland. It is a V- or Y-shaped organ with a hypoechoic cortex and a hyperechoic inner medulla
Clinical Uses of Endoscopic Ultrasound in the Foregut
Esophageal Disease
The esophagus is the easiest part of the gastrointestinal tract to evaluate with EUS and thus plays an important role in the staging of esophageal cancer particularly with the increasing use of neoadjuvant therapy. EUS allows accurate assessment of depth of invasion and the nodal status. However, its role in identifying metastatic disease is limited. The esophagus has five ultrasonographic layers, namely, mucosa, muscularis mucosa, submucosa, muscularis propria, and adventitia (Fig. 11.8). Puli et al. conducted a meta-analysis and reported a sensitivity of 81–90 % and specificity of 99 % for T staging. The accuracy of T staging with T4 tends to be higher in comparison to T1 [7]. In advanced tumors where the lumen is too narrow to allow examination with the echoendoscope, a mini-ultrasound probe can be used through the endoscope to assess the depth of the invasion. EUS can identify local lymph nodes including paraesophageal, paratracheal, subcarinal, and aortopulmonary groups. In addition, it allows biopsy of any suspicious nodes. EUS has a sensitivity and specificity of 84.7 and 84.6 %, respectively, which improves to 96.7 and 95.5 % with the use of FNA [7]. EUS allows visualization and biopsy of metastatic lymph node, particularly celiac adenopathy with sensitivity of 67 % and specificity of 98 %. EUS can also be helpful to visualize much of the liver, with exception to the subdiaphragmatic part of the right lobe.
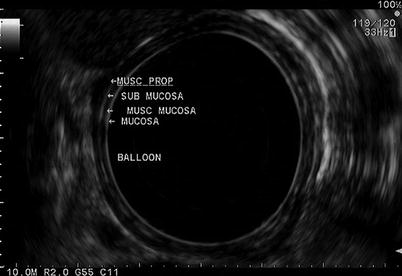

Fig. 11.8
A radial view of the esophageal layers is obtained with a radial echoendoscope. Beginning from the lumen (balloon) and extending outward, the mucosa, muscularis mucosa (musc mucosa), submucosa, and muscularis propria (musc prop) layers can be seen. The mucosal layers (mucosa and submucosa) are hyperechoic, while the muscular layers (muscularis mucosa and muscularis propria) are hypoechoic
Gastric Disease
Similar to the esophagus, EUS plays an important role in the management of gastric cancer. It can help in determining if the patient requires neoadjuvant treatment and also if the patient is a candidate for endoscopic resection. The overall accuracy of EUS for T staging is 75 %, and the accuracy tends to be higher for more advanced disease. It has an accuracy of 77 % for T1, 65 % for T2, 85 % for T3, and 79 % for T4 [8]. However, EUS is 86 % sensitive and 91 % specific in differentiating early “T1/T2” from advanced “T3 and T4” lesions [9]. Higher-frequency (12–20 MHz) ultrasound probes have lower depth of penetration and lower accuracy to stage advanced lesions. Nevertheless, higher frequencies have a higher accuracy for differentiating smaller lesions, which can be particularly helpful for tumors that appear to be amenable for endoscopic resection. Endoscopic ultrasound has a sensitivity of 74 % and specificity of 80 % in lymph node staging and allows biopsy of any clinically suspicious lymph nodes. Suspicious lymph nodes are usually hypoechoic, round, and larger than 10 mm in size (Fig. 11.9). EUS can also identify ascites and can evaluate many parts of the liver.
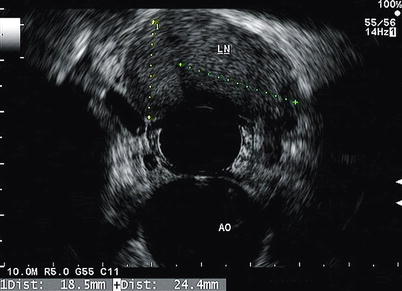

Fig. 11.9
An enlarged lymph node (LN) is seen in the mediastinum. The aorta (AO) can also be seen at 6 o’clock
Biliary Disease
Transabdominal US is the gold standard for evaluation of gallbladder stones. However, it can miss small stones. In patients with suspected gallbladder stones and a nondiagnostic transabdominal US, EUS can be used to evaluate for occult cholelithiasis given its higher-frequency resolution and its closer proximity to the biliary system as compared with transabdominal US. Similarly in patients with acute idiopathic pancreatitis, EUS can be used to rule out occult cholelithiasis or microlithiasis. EUS has also emerged as a minimally invasive procedure for the evaluation of choledocholithiasis, especially among patients with intermediate probability of common duct stones. In this setting, transabdominal US is not very sensitive, and ERCP is associated with a small but not insignificant risk of serious complications. Because of these potential complications such as pancreatitis, cholangitis, perforation, and hemorrhage, ERCP should ideally be reserved for patients with proven common bile duct stones. EUS allows detection of common bile duct stones with sensitivities similar to MRCP and even ERCP in some studies. The exam is usually started with the echoendoscope in the long position in the duodenal bulb. The scope is advanced to the superior angle of the duodenal bulb and the tip is deflected downward. The transducer is then moved slowly along the course of the gallbladder using torque and tip deflection as needed to image the body, fundus, and neck of the gallbladder. The normal gallbladder appears as a large fluid-filled (anechoic) structure with a thin-layered wall. The common bile duct, common hepatic duct, and portal vein are also seen in their long axis with the scope in this position. Doppler can be used to distinguish blood vessels such as the portal vein and gastroduodenal artery from the bile ducts. The scope can then be placed in the short position at the level of the papilla similar to the endoscope position when performing ERCP. This allows identification of the bile duct in the periampullary area. The bile duct can also be followed proximally to the gallbladder and also the level of the bifurcation [10].
EUS can also be used in the management of biliary obstruction. ERCP remains the gold standard for drainage of biliary obstruction caused by benign or malignant diseases. However, conventional ERCP may be difficult in cases of impacted stone at the ampulla, ampullary stenosis, or ampullary carcinoma. In such cases, other options, which are considered to be more invasive and morbid, would include PTC or CBDE. In addition, ERCP may be impossible in patients who have altered anatomy due to previous gastric surgery or duodenal bulb obstruction. More recently, EUS has been used to drain the biliary tree as a safe valid alternative to other options with adequate clinical and technical success. EUS-guided cholangiogram was first reported by Wiersema in 1996 [11] and EUS-guided drainage was first reported by Giovannini in 2001 [12]. A therapeutic linear echoendoscope with a large working channel is used to access and stent the biliary tree using a technique similar to EUS-guided cystgastrostomy. The CBD is visualized through the duodenal bulb or the left hepatic duct is visualized through the stomach by ultrasound. Doppler is used to avoid puncturing a vessel. A 19-gauge needle then is used to puncture the duct and bile aspirated. Afterward, contrast is injected under fluoroscopy and a cholangiogram is performed to delineate the biliary tree and area of obstruction. This is followed by advancing a guidewire into the hepatic duct. The needle then is removed, and dilator is inserted to dilate the tract. A double-pigtail or metallic stent is inserted across the area of obstruction [13]. Early complications include bleeding, right hepatic duct obstruction, cholangitis, and pneumoperitoneum. Late complications include stent migration and cholangitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







