Fig. 16.1
Schematic representation of main bariatric procedure. VBG vertical band gastroplasty, SG sleeve gastrectomy, RYGB Roux-en-Y gastric bypass, BPD-DS biliopancreatic diversion with duodenal switch (reproduced with permission from [73])
It is important for endoscopists to understand the different types of operation, the resulting anatomical alterations, and the different complications that can arise following these procedures.
16.2.1 VBG: Vertical Banded Gastroplasty
In this restrictive procedure, both band and staples are used to create a small stomach pouch. The surgery isolates a small section of the stomach for processing food, limiting the size of meals to approximately 1 oz, and slows digestion by forcing the food to pass through a restrictive ring and thence onto the remainder of the gastrointestinal tract. The isolated pouch is usually made using the lesser curvature of the stomach, virtually bypassing the gastric fundus; a trangastric window is made 6–8 cm below the His angle using a circular stapler, and a linear stapler is placed to create a pouch of 30 ml. The narrow outlet of 10–11 mm is surrounded by a non-distensible collar of polypropylene mesh of PTFE (polytetrafluoroethylene) or a silicon ring, to avoid enlargement. The gastric pouch is small generally 15–30 ml in volume. The expected endoscopic findings after VBG consist of a clean gastric channel 6–8 cm long, with a rosette at the distal end and snug passage of an 11-mm scope without difficulty. Special care should be made to examine the pouch and suture line for fistulas and ulcerations. Retroflexion of the tip of the endoscope in the distal stomach allows inspection of the caudal aspect of the staple-line partition and the remainder of the gastric fundus.
16.2.2 RYGB: Roux-en-Y Gastric Bypass
Roux-en-Y gastric bypass involves stapling the upper stomach into a small proximal 15–30-ml pouch along the lesser curvature, attached to the jejunum through a narrow (11 mm) anastomosis, bypassing a large part of the stomach and duodenum. There are two anastomoses: gastrojejunal and jejunojejunal.
The gastric pouch is small generally 15–30 ml in volume. The expected endoscopic findings after RYGB include a normal esophagus and gastroesophageal junction. The size of the gastric pouch varies. The pouch may have several different configurations and may be long and narrow or shot and wide. Special care should be made to examine the pouch and suture line for fistulas and ulcerations. The gastrojejunal stoma should be easily visible within several centimeters of the gastroesophageal junction and should be carefully examined. The width of the anastomosis is generally 10–12 mm in diameter. Beyond the anastomosis, a short, blind limb is often visible alongside the efferent jejunal limb. The jejunojejunal anastomosis can sometimes be reached with an upper endoscope, depending on the length of the Roux limb. It should be noted that the length of the Roux limb after an RYGB can vary significantly from standard Roux limbs created for nonbariatric procedures and can range from 50 to 150 cm. The distal or excluded stomach cannot be visualized in the absence of a fistula with a regular gastroscope.
16.2.3 SG and BPD-DS: Sleeve Gastrectomy and Biliopancreatic Diversion with Duodenal Switch
Biliopancreatic diversion with duodenal switch (BPD-DS) includes both restrictive and malabsorptive components. Restriction is incurred by a sleeve gastrectomy (SG) in which the greater curvature or left side of the stomach is surgically removed. The surgeon accomplishes this resection by a linear stapler, starting at a point about 5–8 cm to the left of the pylorus along the greater curvature. The stapler line continues vertically upward, roughly paralleling the lesser curvature, until the upper edge of the stomach is reached near the angle of His.
The malabsorptive component of the BPD-DS is a result of two anatomic changes: first, the overall length of the alimentary limb is decreased (duodenal–ileal anastomosis or gastric–ileal anastomosis if the BPD follows a partial distal gastrectomy). Second, the intermixing of bile and pancreatic juices is limited to the distal portion of the alimentary limb, generally 50–100 cm in length (ileoileal anastomosis).
There are no real anastomoses in the SG but only a suture line alongside the grater gastric curve, while in BPD-DS two anastomoses are created.
On endoscopic evaluation of SG, the stomach will appear to be quite long and narrow. The fundus is absent. The stomach is limited in expansion by a staple line that parallels the lesser curvature. The staple line should be examined for defects and ulcerations.
In BPD-DS, immediately below the pylorus, the proximal anastomosis will be traversed. For unclear reasons, the formation of strictures at the duodenoileostomy is rare.
16.3 Anastomotic Complications: Incidence, Diagnosis, and Endoscopic Treatment
16.3.1 Stomal/Marginal Ulcer
Marginal ulcers are typically seen 1–6 months after surgery and may present with abdominal pain, bleeding, or nausea, although they may also be asymptomatic. Ulcerations on the gastric side of the anastomosis (stomal ulcers ) (Fig. 16.2) or on the jejunal surface of the anastomosis (marginal ulcers) are thought to arise from a number of factors, including local ischemia, staple-line disruption, effects of acid on exposed intestinal mucosa, and the presence of staples or suture material [5]. Factors that increase the risk of marginal ulcers include smoking and nonsteroidal anti-inflammatory drug use, whereas proton pump inhibitor use appears to decrease the risk. The true incidence of a marginal ulcer after an RYGB is uncertain, with reports that range from 1 to 36 % [6, 7].
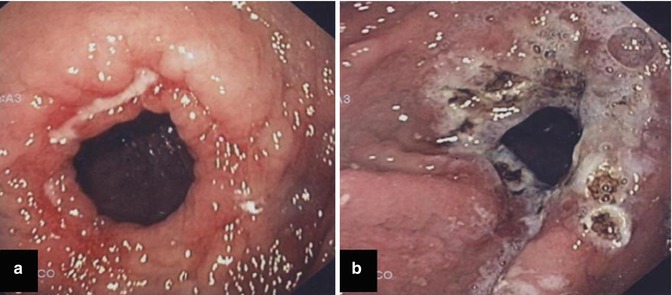

Fig. 16.2
Dilated anastomosis following gastric bypass (a), 2 years after surgery. Note the marginal ulceration at the gastric side. In (b) the same patient after APC treatment
Marginal ulcer can coexist with gastrogastric fistula (VGB and RYGB) or suture leak (SG and BPD-DS). In a large series of 1292 consecutive divided Roux-en-Y gastric bypass with 17.6 months of follow-up [8], 15 patients (1.2 %) presented for endoscopic evaluation and were found to have gastrogastric fistulas. Of these, 12 (80 %) complained of nausea, vomiting, and abdominal pain. Four patients (27 %) presented because of failure in losing weight. On endoscopic examination, eight patients (53 %) were found to have a coexisting marginal ulcer.
The cause of true stomal ulcers is thought to be ischemic in nature, whereas the cause of marginal ulcers is poorly understood [9]. Multiple mechanisms have been proposed to explain marginal ulcers. Local ischemia, larger pouch size leaving retained parietal cells that produce gastric acid, acidic gastric secretions poorly tolerated in the jejunum, NSAID use, alcohol use, smoking, a coexisting gastrogastric fistula, and the presence of a foreign body such as nonabsorbable suture material have been implicated [10–13]. To evaluate the predictors of endoscopic findings, a retrospective review of 1001 RYGB [4] showed that smoking, NSAID use, and abdominal pain predicted the presence of marginal ulcers at endoscopy; smoking and NSAID use also predicted staple-line dehiscence. Age, gender, surgical technique, and surgeon experience did not predict abnormal findings at endoscopy.
Refractory ulcers should raise concern for the presence of a gastrogastric fistula.
Moreover, also time of presentation from surgery can predicted findings for the presence of stomal ulcers and stomal stenosis. In another RYGB large series [14], presenting more than 6 months after surgery was associated with a lower likelihood of stomal ulceration or stenosis. In contrast, presenting after 6 months was associated with a greater likelihood of staple-line dehiscence [14].
In treating marginal ulcer, it is advisable to remove nonabsorbable sutures when visible intraluminally to assist with healing, prevent gastrogastric fistulas, and relieve chronic abdominal pain in patients who underwent bariatric surgery. Long-term treatment with oral proton pump inhibitors therapy, along with antibiotics for coexisting H. pylori infection, has led to healing of fistula. The role of Helicobacter pylori is still not clear. In one study, marginal ulcer and associated gastrogastric fistula responded to a combination of PPI therapy and fibrin glue injections [15]. Healing times for ulcer resolution vary from 8 weeks to 6 months but were longer in the presence of an untreated or undiagnosed fistula [16].
Bleeding duodenal ulcers have been rarely reported following Roux-en-Y gastric bypass [17]. Early postoperative bleeding in RYGB most likely originates at the gastrojejunostomy.
Endoscopic management of early postoperative intraluminal bleeding is challenging and controversial due to the risk of dehiscence and perforation at the surgical anastomosis. Endoscopy is usually not necessary because bleeding is mild and self-limited in most cases but should be considered in patients in whom bleeding is severe (hemodynamic instability and/or ≥2 g drop in hemoglobin) or when rebleeding occurs. If endoscopy is performed, air insufflation should be minimized to prevent disruption of the anastomosis. Close communication with the surgeon is essential. Data of endoscopic findings and management of early postoperative bleeding show that most patients (20/27; 74 %) underwent endoscopy in an operating room and were endotracheally intubated (19/27; 70 %). Bleeding stigmata seen at the gastrojejunostomy included active oozing (48 %), visible vessel (26 %), and adherent clot (26 %). Endoscopic therapy was performed in 85 % of patients and included epinephrine injection, heater probe coagulation, combination epinephrine injection and thermal coagulation, and hemoclip placement in 3 (13 %), 4 (17 %), 14 (61 %), and 2 (9 %) patients, respectively. Hemostasis was achieved in all patients, but 5 (17 %) patients required surgery to control hemorrhage and complication occurred (pulmonary aspiration and perforation) [18].
On occasion, postoperative nausea and vomiting may lead to a bleeding Mallory–Weiss tear, which can be managed endoscopically.
Summarizing, stomal and marginal ulcer can successfully be treated conservatively with PPI; bleeding ulcers can be endoscopically treated in more than 2/3 of the case. Success of the endoscopic treatment depends mainly on the severity of the bleeding.
16.3.2 Stomal Stenosis
Stomas are generally 10–12 mm in diameter and stenosis is defined as a diameter <10 mm.
Stenosis of the stoma has to be divided into the immediate postoperative, early postoperative (<3 months), and late postoperative (>3 months) period, as edema or edema with early scar formation respond well to dilation and the outcome in late scarring is rather poor. Stomal obstruction in the initial postoperative period has been simply solved by waiting to see whether the stomal edema and swelling subside after replacement of a nasogastric tube [19]. Stenosis occurring later is believed to result from fibrosis or an inflammatory reaction (occurring around the band in VGB).
Stomal stenosis occurs in as many as 4.73–27 % of patients undergoing RYGB [20, 21]. These patients typically present with dysphagia, nausea with vomiting, or early satiety as previously noted. The primary endoscopic intervention is balloon dilation up to 15–18 mm, which has been associated with a greater than 93 % success rate in symptom resolution and subsequent weight loss [22, 23] (Fig. 16.3). Dilation with Savary-Gilliard bougie (Cook Endoscopy; Winston-Salem, NC, USA) may be considered and is an effective intervention. In one review, both methods required 2–3 sessions of therapy, with a complication rate of 3 % [24]. Endoscopic fluoroscopy-guided balloon dilation has been demonstrated safe, effective, and durable [25–28].
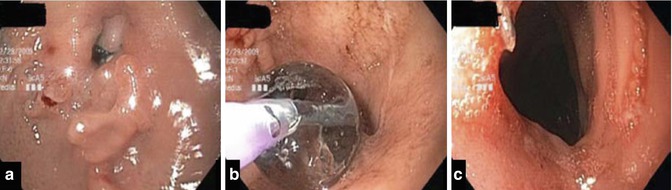

Fig. 16.3
Stomal stenosis following gastric bypass (a). In (b) CRE balloon dilatation and in (c) the anastomosis after treatment
Gradual dilation over a few sessions is likely the best; dilatation should not be performed to >15 mm, as this may be associated with future weight gain. For symptomatic patients presenting with refractory vomiting, thiamine repletion should be considered early and before exogenous glucose administration to prevent the precipitation of Wernicke encephalopathy [29].
16.3.3 Gastrogastric Fistula
In VBG and RYGB, the gastric suture line can present one or more dehiscences allowing an opening communication between the pouch and the excluded stomach, known as a gastrogastric fistula .
Because of the communication, it is a particular type of suture dehiscence, always presenting on endoscopy as fistula completely or partially re-epithelized and so far different from other anastomotic leaks.
Most large series report that gastrogastric fistulas occur in 1.2–1.8 % of patients undergoing gastric bypass [30]. However, incidence rates from zero to as high as 46 % have been reported, with substantial improvements in recent years because of modifications in the surgical technique [31].
Usually patients complain for weight regain or for not being able to lose weight.
Because of the high rate of morbidity and mortality associated with surgical revision of gastrogastric fistulas, initial treatment has evolved from surgical interventions to endoscopic management, with variable success. Reported endoscopic techniques (Fig. 16.4) include the use of fibrin glue sealants [32, 33], insertion of a Surgisis fistula plug (Cook Surgical, Inc, Bloomington, IN, USA) with or without a self-expanding stent [34], endoluminal stent placement [35], the use of mucosal suturing devices for tissue apposition [36], and local debridement following argon plasma coagulation [37]. The optimal method of treatment is unknown, as comparison studies and randomized controlled trials are lacking.
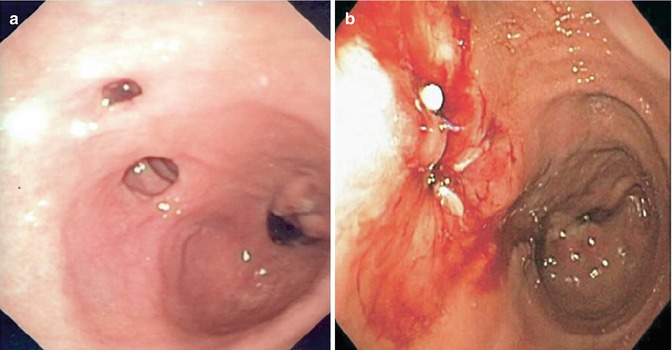

Fig. 16.4
Gastrogastric fistula following VBG (a). In (b) and endoscopic view after argon plasma coagulation (APC) and fibrin glue
16.3.4 Anastomotic Rupture/Dehiscence/Leaks
Published incidence rates for leaks following bariatric surgery range from 0.4 to 26 %, and leaks are associated with a mortality rate of 1.5 % [38].
Gastric leaks are potentially serious complications of bariatric surgery and occur in 1–6 % of patients in gastric bypass and 0.7–4.6 % in sleeve gastrectomy [39, 40]. Next to pulmonary embolus, intra-abdominal sepsis secondary to leaks is the most serious life-threatening complication associated with bariatric surgery. The potential causes of leaks are multiple: tension on the anastomosis, staple or stapler malfunction, suture or staple-line seepage, poor surgical technique, obstruction, hypovascularization, and hematomas [41].
Leaks require early recognition of symptoms, detection, and prompt treatment to prevent loss of life. Clinical manifestations include tachycardia, fever, nausea, vomiting, and abdominal or chest pain.
In RYGB most leaks occur at the gastrojejunal anastomosis, with nearly all the rest occurring in the remnant (excluded) stomach; leaks from the jejunojejunal anastomosis are less common but do occur and usually require reoperation. In sleeve gastrectomy, the critical areas for leak are the top of the suture line and the transition point between sequential cartridge.
The primary diagnosis is done using radiologic techniques: upper gastrointestinal imaging (UGI) and CT scans. Because of patient obesity, both of these tests have limited diagnostic sensitivity [42, 43]. Despite this limitation, radiologic evaluation is important in the early detection of leaks in the postoperative setting [44]. The primary diagnostic tool used to assess for postoperative leaks is a UGI study with an oral water soluble contrast. This study is typically performed on postoperative day 1 or 2 and has shown variable sensitivity in detecting leaks (many leaks occur after a UGI study).
Management of bariatric leaks has traditionally consisted of drainage, antibiotics, and specialized nutrition. In patients with hemodynamic instability, a surgical approach is preferred. In recent years there has been an increase in the nonoperative management of leaks after bariatric surgery as most leaks are well contained and do not require operative control.
There is little role for an endoscopy in the presence of known leaks or fistulas in the early postoperative period. An endoscopy can be considered if the patient is clinically stable, there is uncertainty of the diagnosis, or if there is a planned endoscopic intervention [35, 45].
Chronic fistulas may be found in the presence of marginal ulcers, and patients may present with nausea, vomiting, epigastric pain, and weight gain.
Recent reports demonstrate that endoluminal interventions are effective in healing anastomotic breeches. Multiple investigators are reporting the successful placement of covered endoluminal stents and the initiation of oral nutrition leading to recovery from this postoperative complication [46–48].
Endoscopic therapy for postoperative fistulas has been performed also by using fibrin glue injection (Fig. 16.5) [49, 50] or self-expanding stents [51–53] (Fig. 16.6). A possible adjunct in managing postsurgical leaks involves minimally invasive techniques using stents placed with endoscopic and fluoroscopic guidance. Covered stents can be placed in the bowel lumen at the site of the leak in a minimally invasive fashion. Stents offer several treatment advantages that can simplify surgical management of postoperative leaks. A stent prevents or greatly diminishes further peritoneal contamination by excluding the leak site from enteral secretions. This in turn is thought to promote and accelerate leak healing. Stent placement results in a rapid improvement in abdominal pain as a result of decreased peritoneal contamination. Shielding of the leak site also permits nutrition to be given orally in many cases. Parenteral nutrition is seldom necessary.
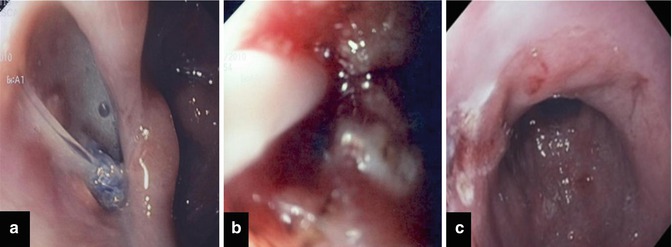
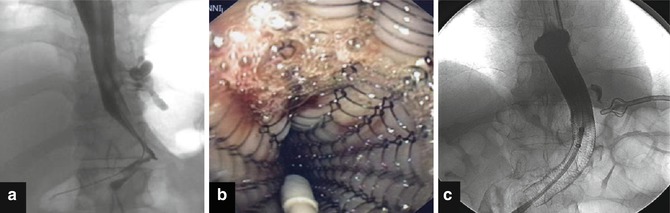

Fig. 16.5
Suture leak following sleeve gastrectomy. In (a) endoscopic view of the fistula. At the bottom it is possible to recognize a surgical stitch. In (b) endotherapy with APC and fibrin glue application. In (c) endoscopic appearance after 1 month after endoscopic treatment

Fig. 16.6




Endoscopic therapy for postoperative fistulas with self-expanding stent. In (a) fluoroscopic appearance of the fistula before treatment. In (b) endoscopic view during self-expanding stent release and in (c) post placement fluoroscopic control
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








