Endoscopic therapy for Barrett’s esophagus is feasible and likely to decrease the future risk of development of esophageal adenocarcinoma. The most commonly used therapy is radiofrequency ablation, which has been shown to produce reproducible superficial injury in the esophagus. Other thermal therapies include multipolar coagulation, argon plasma coagulation, and thermal laser therapy. The other end of the ablative spectrum includes cryotherapy, which involves freezing tissue to produce mucosal necrosis. Photodynamic therapy has been used to photochemically eliminate abnormal mucosa. Endoscopic therapy has been demonstrated to be effective in high-risk situations such as Barrett’s esophagus with high-grade dysplasia.
Key points
- •
Endoscopic eradication therapy is considered a safe, effective, and durable treatment strategy for Barrett’s esophagus complicated by high-grade dysplasia and early esophageal neoplasia.
- •
The endoscopist should be familiar with both mucosal resection and ablation techniques, and their respective indications.
- •
Patients should be enrolled in a comprehensive surveillance program that continues following endoscopic eradication therapy.
- •
Recurrent dysplasia can be approached endoscopically as long as appropriate staging is performed.
Background
Endoscopic ablation therapy has evolved from being a possibility in patients who could not undergo esophagectomy to a standard of care for the treatment of early esophageal neoplasia (EAC). Its increasing efficacy and decreasing morbidity through the years have made this approach the preferred treatment for Barrett’s esophagus (BE) with high-grade dysplasia (HGD) and early EAC. Several treatment modalities are currently available, which can be broadly classified as mucosal resection and ablative techniques. These modalities are usually used in combination and as part of a treatment program that requires endoscopic surveillance.
Background
Endoscopic ablation therapy has evolved from being a possibility in patients who could not undergo esophagectomy to a standard of care for the treatment of early esophageal neoplasia (EAC). Its increasing efficacy and decreasing morbidity through the years have made this approach the preferred treatment for Barrett’s esophagus (BE) with high-grade dysplasia (HGD) and early EAC. Several treatment modalities are currently available, which can be broadly classified as mucosal resection and ablative techniques. These modalities are usually used in combination and as part of a treatment program that requires endoscopic surveillance.
Risk stratification of Barrett’s esophagus
Risk stratification begins with a detailed examination of the Barrett’s mucosa under white-light endoscopy. Irregularities in the mucosa are targeted with biopsies or endoscopic mucosal resection (EMR) because these sites are more likely to contain neoplasia. Tissue sampling is also performed at 4-quadrant intervals over the entire BE segment to detect dysplasia that may not be apparent under endoscopic evaluation. Advanced imaging technologies such as narrow-band imaging, confocal endomicroscopy, and optical coherence tomography can be used to enhance detection of dysplasia, but are currently not a substitute for thorough examination under high-resolution white-light endoscopy.
Endoscopic eradication therapy should be considered in patients with the highest risk of progression to invasive EAC whereby metastatic lymphadenopathy has been excluded. Risk stratification is currently based on histopathologic evaluation for grade of dysplasia, which is unreliable because of the lack of agreement among pathologists regarding the exact degree of dysplasia. One indicator that low-grade dysplasia is more likely to progress is agreement between 2 or more pathologists. The risk with nondysplastic BE is about 0.18% per year, less than half the risk estimated only 5 years ago. The decrease in cancer risk appears to be related to more reports from large population-based databases. The absolute risk of EAC increases in proportion to the grade of dysplasia with HGD, carrying a 30% 5-year cancer risk. The risk of metastatic lymphadenopathy is proportional to the depth of invasion, and is low (<5%) for neoplasia confined to the mucosa (intramucosal adenocarcinoma, stage T1a). Other risk factors for potential metastatic disease with early T1a cancer are evidence of lymphovascular invasion and high-grade malignancy. The most reliable technique to obtain this information is EMR, which can be used to diagnose, stage, and treat early cancer.
Theory of ablation
It is important to realize that it is unclear why ablation therapy results in squamous regeneration. The current belief is that intestinal metaplasia occurs because of chronic injury to the esophageal mucosa with the production of cytokines such as interleukin (IL)-1β, IL-6, and IL-8, which increase expression of transcription factors that promote intestinalization such as CDX2 and bone morphogenetic protein 4 (BMP4) ( Fig. 1 ). These factors have been shown in animal models to act on gastric glandular stem cells to produce an intestinal phenotype that migrates proximally into the esophagus. These cytokines are also produced by adipocytes, and can be a partial biological explanation of how obesity promotes BE.
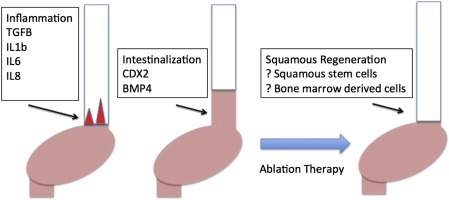
The elimination of BE caused by endoscopic injury and secondary production of squamous mucosa is unclear. Studies have found that the squamous mucosa that regenerates does not contain genetic abnormalities similar to the intestinal mucosa, indicating that there may well be a different cell of origin for these squamous stem cells. It is thought that, similar to keratinocytes in the cutaneous skin, the stem cells of origin of these squamous cells are the interpapillary basal cells. This assumption would imply that squamous regeneration should occur from neighboring squamous mucosa, and confirms early observations in ablation therapy that if 3 areas of squamous mucosa surrounded the area of treated columnar tissue, squamous regeneration was more likely than if the area was not in contact with any squamous mucosa. However, it has been observed that squamous islands appear to form if deep injury to the mucosa has occurred, as is seen with mucosal resection. It has also been found that bone marrow stem cells migrate to areas of deep tissue wounds throughout the body, and it is possible that these isolated patches of squamous mucosa are the result of bone marrow–derived squamous stem cells. There is little clinical evidence that substantiates this hypothesis, and it would require a very unusual situation whereby a male patient undergoes ablation after a bone marrow transplant from a female to permit lineage tracing of the bone marrow donor.
Endoscopic mucosal resection
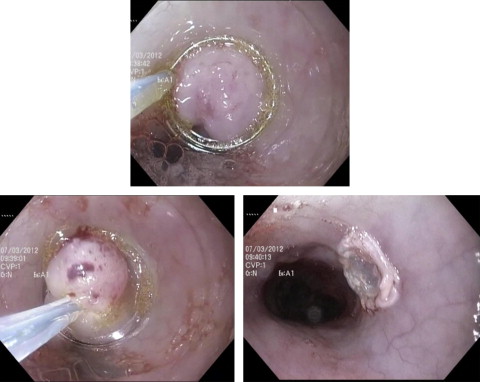
EMR refers to the use of a standard polypectomy technique in flat mucosa for the purposes of resecting suspicious and dysplastic lesions. Despite its designation as a “mucosal” resection technique, EMR actually extends into the submucosa. Hence, it is not uncommon that EMR is sometimes referred to as endoscopic resection (ER) ( Figs. 2 and 3 ).
In the setting of appropriate risk stratification, patients with HGD and early EAC may be offered the choice to undergo EMR instead of esophagectomy. The risk of advanced malignancy depends on the depth of neoplastic luminal involvement. As such, EMR should only be attempted in patients with a low risk of locally advanced EAC or metastatic lymphadenopathy. EMR has emerged as an indispensable tool in endoluminal therapy for dysplastic BE and early EAC. Its use has increased because it accomplishes the basic prerequisites of surgical therapy for malignancy in a minimally invasive manner. EMR also offers a dual advantage for tissue diagnosis aside from its therapeutic use. Studies have demonstrated that pathologists achieve improved interrater agreement and accuracy for detecting HGD and early EAC in comparison with traditional biopsies.
In the United States there are 2 commercially available devices for esophageal EMR, either as a cap-type or multiband device. The cap technique uses a hard or soft plastic cap and snare for ligation of the target mucosa. The cap also comes in flat and oblique configurations, with the oblique cap capable of removing more tissue. The cap technique requires a snare to be fitted on the end of the cap, and requires experience in placing it correctly. On the other hand, the multiband technique evolved as a modification of the variceal banding device. The banding device is available with 6 prefitted bands. With this kit the dysplastic mucosa is suctioned deep into the plastic cap, at which time the band is deployed (see Fig. 2 ). This band creates a pseudopolyp of tissue to prevent luminal perforation. Once the tissue of interest has been captured, a snare is used to resect the tissue.
The initial step of EMR is lifting the target mucosa with the use of a dilute epinephrine injection (1:200,000). The mucosal lift facilitates separation of the mucosa from the submucosa. This maneuver is crucial in preventing perforation during resection, especially when using the cap technique. Once the resection is completed using of the snare, the EMR tissue should be retrieved for histopathologic evaluation to determine the grade of dysplasia and involvement of the margins.
Retrospective studies have shown that the efficacy of EMR is comparable with that of esophagectomy in the treatment of localized EAC. Complete eradication of dysplasia (CR-D) has been reported to be greater than 90% with or without subsequent ablation therapy. EMR is well tolerated and has a low risk of complications under the hands of experienced endoscopists. Complications such as bleeding or perforation occur in fewer than 4% of cases, and these can be controlled with the use of hemoclips or stents.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



