INTRODUCTION
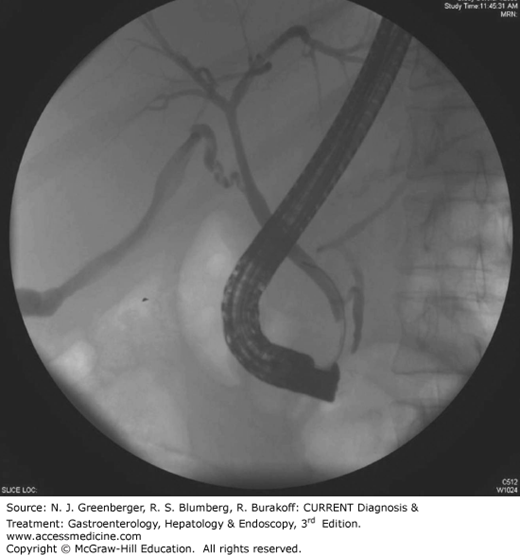
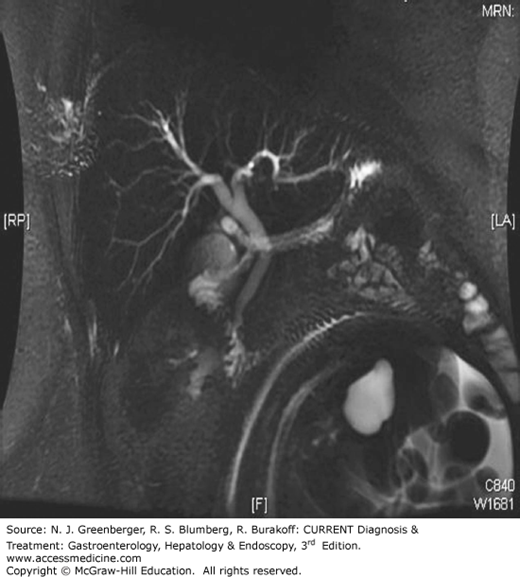
Endoscopic retrograde cholangiopancreatography (ERCP) (Figure 36–1) is a combined endoscopic and fluoroscopic procedure that was introduced in the early 1970s to allow access to the biliary and pancreatic ductal systems through their openings at the major and minor duodenal papillae. ERCP has evolved from a purely diagnostic technique, performed by a few, into a complex set of procedures integrating diagnosis and therapy for a wide variety of pancreatobiliary disorders offered in all major medical centers. ERCP requires dedicated training in order to acquire the range of techniques that include endoscopic papillectomy, sphincter of Oddi manometry (SOM), biliary sphincterotomy, pancreatic sphincterotomy, stone removal, tissue sampling, placement of plastic and metallic stents, cholangioscopy, pancreatoscopy, and drainage of pancreatic fluid collections. Although it has become a highly successful therapeutic modality, ERCP also carries an overall morbidity of 7% that includes pancreatitis (4%), hemorrhage (1%), cholangitis (1%), perforation (0.5%), and death (0.1%). With the advent of magnetic resonance cholangiopancreatography (MRCP) (Figure 36–2) which safely and noninvasively images the pancreatic and biliary tracts and endoscopic ultrasound, the need for purely diagnostic ERCP has appropriately diminished considerably while the demands for therapy have grown.
DISORDERS OF THE MAJOR DUODENAL PAPILLA
Small intestinal tumors represent 2.4% of all gastrointestinal malignancies and over half of these are found in the duodenum. Duodenal adenomas may occur sporadically or in 50–100% of patients with familial adenomatous polyposis (FAP) and are most commonly in the periampullary location. Papillary adenomas may be detected as an incidental finding during upper endoscopy performed for another indication or may manifest with recurrent pancreatitis, weight loss, or biliary obstruction. The frequency of carcinoma in papillary adenomas ranges from 15% to 60%.
Adenomas of the papilla follow the adenoma–carcinoma sequence similar to that seen in the colon; thus, resection of these lesions is recommended. Traditionally, the approach was surgical resection by pancreaticoduodenectomy because the presence of malignancy could not be completely excluded based on preoperative biopsies. Due to significant morbidity, even with improvements in surgery, endoscopic resection is currently the treatment of choice. Since the first reports of endoscopic resection of the papilla in the early 1990s, endoscopic papillectomy or ampullectomy has gained wider acceptance as a less invasive therapy.
Accurate preoperative diagnosis and staging of papillary lesions is essential. ERCP with biopsies and endoscopic ultrasound are the current accepted approach for diagnosis and staging of local invasion and assessment of lymph node status. Invasive cancer is a contraindication to endoscopic resection. Inoperable ampullary cancer causing biliary obstruction is best treated by sphincterotomy if feasible or by placement of a self-expanding metal stent.
The technique of ampullectomy involves inspection of the papilla with a side-viewing duodenoscope, and ERCP to define any intraductal tumor extension and to opacify the ducts with contrast to aid in identifying the ductal orifices after the procedure is completed. Most experts resect the papilla and some surrounding normal mucosa en bloc with an electrocautery polypectomy snare and retrieve the specimen for histologic examination. Residual adenomatous tissue at the margins of the resection site can be ablated with contact thermal or noncontact argon plasma coagulation. After resection, a short polyethylene pancreatic stent is placed to minimize the risk of post-procedural pancreatitis, and many endoscopists also place a biliary stent if a sphincterotomy has not been performed (Plates 88, 89, 90). The stents are endoscopically removed about 1 month later, and the site is inspected for residual adenoma. Surveillance endoscopy with a duodenoscope is offered every 6–12 months.
The sphincter of Oddi is a complex muscular structure that surrounds the distal pancreatic duct, bile duct, and ampulla of Vater. This sphincter mechanism lies mostly within the duodenal wall and measures 6–10 mm in length. Functionally, the sphincter of Oddi is independent from the duodenal smooth muscle system. It serves to prevent reflux of duodenal contents into the ductal system and controls the flow of bile and pancreatic juice into the duodenum.
Sphincter of Oddi dysfunction (SOD) describes an abnormality within the sphincter, either motility related (dyskinesia or spasm) or structural (stenosis), and can involve the biliary sphincter, the pancreatic sphincter, or both. In pancreatic-type SOD, patients typically present with episodic pancreatic-type epigastric pain radiating to the back with pancreatic enzyme abnormalities or frank acute pancreatitis. The more common biliary-type SOD occurs postcholecystectomy and patients experience abdominal pain similar to the preoperative pain of suspected biliary origin. Each type of SOD has three subtypes:
Biliary-I patients have biliary-type pain, abnormal liver enzyme values greater than twice normal documented on one occasion, and dilated common bile duct greater than 10 mm diameter.
Biliary-II patients have biliary-type pain but only one or two of the preceding criteria.
Biliary-III patients have only biliary-type pain and no other abnormalities.
Pancreatic-I patients have recurrent pancreatitis or typical pancreatic pain, elevated pancreatic enzymes two times the upper limit, and dilated pancreatic duct greater than 5 mm.
Pancreatic-II patients have pancreatic pain and one or two of the above criteria.
Pancreatic-III patients have only pancreatic-type pain.
Sphincter function can be evaluated by noninvasive methods that include hepatobiliary scintigraphy, ultrasound assessment of pancreatic and bile duct after secretin or cholecystokinin stimulation, and secretin-stimulated MRCP. The gold standard diagnostic test for SOD, however, is manometric assessment of basal sphincter pressure by SOM, which involves the use of solid state or perfusion low-compliance catheters that can measure the biliary and pancreatic sphincter pressures through ports located at the distal end. Multiple station pull-throughs are performed and graphic recording of the pressures are displayed on a dedicated workstation. The patient must be sedated for the procedure, but narcotics and smooth muscle relaxants are usually avoided as they may interfere with the recordings. A basal sphincter pressure greater than 40 mm Hg above duodenal pressure is considered to be abnormal, and sphincterotomy (incision of the intraduodenal portion of the common bile duct or pancreatic duct sphincter muscle) is the current standard treatment of choice for SOD in the appropriate clinical setting.
Symptomatic patients with type I SOD benefit from sphincterotomy (90–100%) regardless of manometry findings. Thus, manometry is not required for this group of patients. In type II SOD, 50–70% of patients with elevated basal sphincter pressure on manometry will benefit from sphincterotomy. In biliary type III SOD patients, not only does manometry not predict response to treatment, but also sphincterotomy does not perform better than sham as seen from a recent randomized sham controlled trial.
The rate of complications is high in patients with suspected SOD undergoing ERCP with pancreatitis being most common and occurring up to 20–40%. The risk of pancreatitis is reduced by temporary stenting of the pancreatic duct with or without administration of rectal indomethacin.
[JAMA and JAMA Network Journals Full Text]
BILIARY SYSTEM
Bile duct stones occur in up to 15% of symptomatic patients with cholelithiasis and in up to 2% of cholecystectomies performed for acalculous biliary disease. Patients may present with abdominal pain of biliary origin, cholangitis (see Chapter 54 on Biliary Emergencies), jaundice, pancreatitis, transient elevation of transaminases, or filling defect with or without biliary ductal dilation on imaging studies such as transabdominal ultrasound, computed tomography (CT), and MRCP. When choledocholithiasis is suspected, therapy is directed toward extraction of the stones from the biliary tree to minimize serious complications such as severe pancreatitis, sepsis, and death. Stone extraction can be achieved endoscopically, surgically, or by the transhepatic approach radiologically.
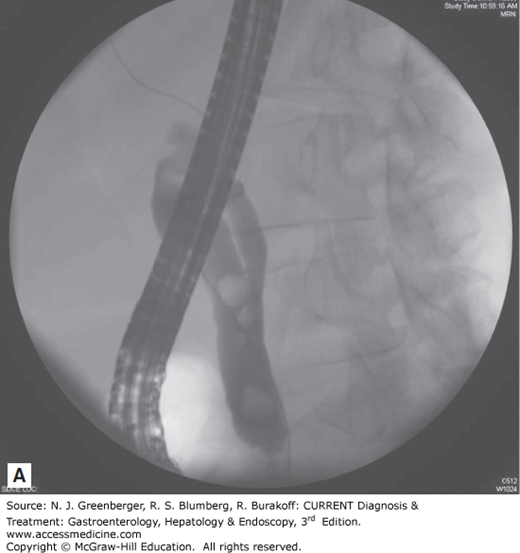
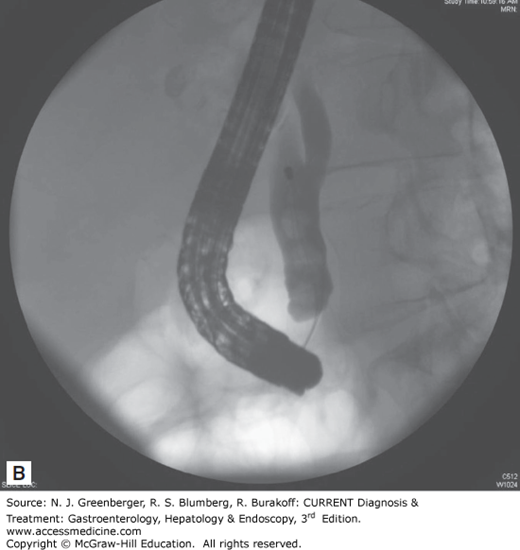
Endoscopic removal of bile duct stones is the treatment of choice in centers with expertise in this technique (Figure 36–3). Since the first descriptions of endoscopic sphincterotomy in 1974, the role of ERCP in the management of bile duct stones has undergone tremendous growth. The standard techniques of stone extraction require access to the bile duct by deep cannulation. Once stones are identified on a cholangiogram, a biliary sphincterotomy is usually performed under direct endoscopic guidance to the maximum extent of endoscopic landmarks using a bow-type sphincterotome, a catheter carrying a cutting electrosurgical wire at its distal end.
An attempt at stone extraction should always be made after biliary sphincterotomy except in urgent, unstable situations. The most commonly used accessories for this purpose are balloon catheters and metal wire baskets (see Figure 36–3). These standard techniques can achieve success in up to 90% of bile duct stones while the remaining 10% of cases require more advanced techniques. In particular, stones larger than 15 mm present a challenge and often require additional endoscopic techniques such as mechanical, electrohydraulic, or laser lithotripsy. When complete stone removal fails, insertion of a biliary stent provides temporary drainage, minimizing the risk of biliary sepsis, before further endoscopic attempts at duct clearance can be made. This stenting approach can lead to over 90% success at stone clearance following two ERCP attempts.
Multivariate analysis of large series of sphincterotomies demonstrated five significant risk factors for complications: suspected SOD, cirrhosis, difficult bile duct cannulation, precut (access) papillotomy, and use of a combined percutaneous-endoscopic procedure. The rate of complications was highest when the indication for the procedure was suspected SOD (22%) and lowest when the indication was removal of bile duct stones within 30 days of laparoscopic cholecystectomy (5%). Endoscopists who performed more than one sphincterotomy per week had lower rates of all complications (8.4% vs 11.1%, P = 0.03) and severe complications (0.9% vs 2.3%, P = 0.01) than those who completed less than one per week.
As an alternative to sphincterotomy, endoscopic balloon dilation of the papilla was introduced in the 1980s. This involves the use of a hydrostatic balloon positioned across the papilla and inflated under high pressure while dilation is monitored fluoroscopically. Endoscopic balloon dilation alone in the treatment of bile duct stones is associated with higher rates of pancreatitis (7.4%) and need for mechanical lithotripsy (20.9%) compared to sphincterotomy (4.3% pancreatitis and 14.8% mechanical lithotripsy). This technique is used regularly in Southeast Asia without high morbidity, but due to increased incidence of pancreatitis in Western populations, balloon dilation of the papilla alone is not recommended as a routine first-line option in the management of bile duct stones.
Large balloon dilation (LBD) of a prior sphincterotomy, however, appears safe and effective especially for large bile duct stones. Two randomized trials reported similar success in removing large bile duct stones with this technique compared to sphincterotomy alone or with mechanical lithotripsy. The latter study suggested lower overall complications, especially cholangitis, with LBD and sphincterotomy.
Hepatolithiasis, the presence of stones in the intrahepatic biliary tree above the hepatic duct confluence, is difficult to treat and poses a major challenge to the endoscopist in countries where this is common. Surgery is often required but rarely solves the problem, since stone recurrence is high.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree