Diagnostic
Primary sclerosing cholangitis
Indeterminate biliary strictures
Sphincter of Oddi manometry
Patient with contraindications to noninvasive imaging
Therapeutic
Common bile duct stones
Benign biliary strictures
Malignant biliary strictures
Primary sclerosing cholangitis
Transpapillary gallbladder drainage
Ampullary adenoma
It is herein worthwhile to comment on two of these indications, namely (1) primary sclerosing cholangitis (PSC) and (2) indeterminate biliary strictures, which can occur within or independent of PSC.
Primary Sclerosing Cholangitis (PSC)
PSC is an idiopathic, chronic cholangiopathy characterized by biliary fibro-inflammation [4, 5], a median liver transplantation-free (LT) survival of 12–15 years [6, 7], and a predisposition to hepatobiliary malignancy, particularly cholangiocarcinoma (CCA) [6, 8, 9]. Despite clinical trials of numerous pharmacologic agents, medical therapy for PSC has yet to be established [4, 5, 10]. Although operative therapy with LT is effective for PSC, it is only performed in specialty centers and in select patients; furthermore, even in appropriate LT candidates, PSC and CCA can recur post-LT [11–13]. The diagnosis of PSC is based on cholangiography demonstrating biliary strictures or characteristic irregularity, liver histology demonstrating sclerosing cholangitis, and clinical features, particularly elevated serum alkaline phosphatase (ALK) [4, 14, 15].
ERC is indicated in the setting of presumed or known PSC when (1) the diagnosis is highly suspected but MRCP is negative or nondiagnostic, (2) cytologic sampling is needed to rule out CCA, or (3) lesions requiring therapeutic intervention are anticipated (e.g., dominant biliary stricture with or without acute hepatobiliary decompensation). Regarding the first of these, it is important that proper techniques be followed to allow acquisition of optimal cholangiographic images, particularly in patients with possible early-stage PSC [16]; for example, complete filling of the intrahepatic ducts is essential to establish the diagnosis and is best achieved when the occlusion balloon catheter is positioned above the cystic duct takeoff. A negative ERC, of note, does not preclude the diagnosis of PSC, as a small fraction of patients will have small duct PSC only [17, 18]. The latter two points, i.e., cytologic sampling and management of dominant strictures, are discussed later in this chapter.
Indeterminate Biliary Strictures
Some biliary strictures cannot be readily classified as benign or malignant on the basis of noninvasive imaging studies, ERC, and/or conventional tissue sampling methods (i.e., wire-guided biliary brushing, intraductal forceps biopsy) [19]. Additional techniques that can be used to evaluate indeterminate strictures include (1) EUS, (2) intraductal ultrasonography [20], (3) probe-based confocal laser endomicroscopy [21–23], and (4) cholangioscopy with or without site-directed biopsy [24, 25]. There are two main methods of cholangioscopy: (1) passage of a cholangioscope through the working channel of the ERC scope and (2) passage of a small caliber, forward-viewing endoscope (4.9 mm) directly into the bile duct. The latter is referred to as direct peroral cholangioscopy and can be technically challenging as the inherent mechanics and angulation do not readily permit a forward-viewing endoscope to be passed directly into the bile duct [26, 27]. However, these forward-viewing endoscopes achieve relatively superior optics (video rather than fiber optic) and have a larger working channel compared to cholangioscopes passed through the working channel of the ERC scope [28].
In a small proportion of patients, the etiology and nature of the stricture may remain unclear despite the aforementioned techniques, in which case the final diagnosis is established during long-term follow-up or by means of surgical or (repeated) nonsurgical tissue sampling.
Therapeutic ERC
The most common therapeutic application of ERC is for management of common bile duct (CBD) stones and palliation of malignant biliary obstruction. However, the range of options for endoscopic therapy of obstructive biliary disease (Table 12.2) has expanded substantially since its initial applications.
Table 12.2
Applications of endoscopic ultrasound in biliary disease
Diagnostic |
Gallbladder |
Stones |
Sludge |
Microlithiasis |
Choledocholithiasis |
Indeterminate biliary strictures |
Ampullary lesions |
Therapeutic |
Transmural gallbladder drainage |
Transgastric drainage of bilomas |
Transgastric drainage of hepatic abscesses |
Biliary drainage |
Rendezvous |
Hepaticogastrostomy |
Choledochoduodenostomy |
Benign Biliary Disease
Bile Duct Stones
ERC is frequently performed in patients with known choledocholithiasis or in those with at least a moderate clinical suspicion of choledocholithiasis [29]. In patients with cholelithiasis and low clinical suspicion of choledocholithiasis, noninvasive imaging studies (MRCP, transabdominal ultrasound) or less invasive EUS may be preferable as an initial step to avoid unnecessary ERC-related complications [30]. In patients with low clinical suspicion of choledocholithiasis in whom cholecystectomy is already planned, intraoperative cholangiography can be performed instead of preoperative ERC; if bile duct stones are identified, stone removal can be undertaken intraoperatively, thus reserving ERC for patients in whom stones are not extracted intraoperatively [30].
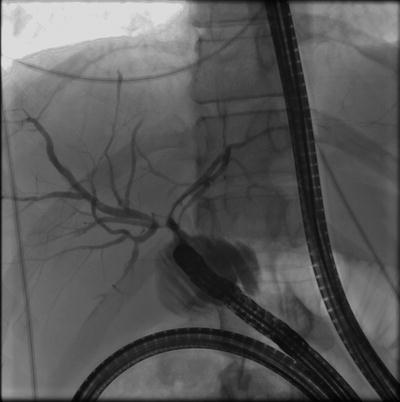
The standard method for stone removal is endoscopic sphincterotomy (ES) to enlarge the caliber of the papilla followed by stone extraction with an occlusion balloon or basket (Fig. 12.1). With this method >95 % of stones <1.5 cm can be removed successfully when performed by experienced endoscopists (and in the absence of underlying strictures or altered bilio-enteric anatomy) [31]. Larger stones may require more advanced techniques, which are discussed later.
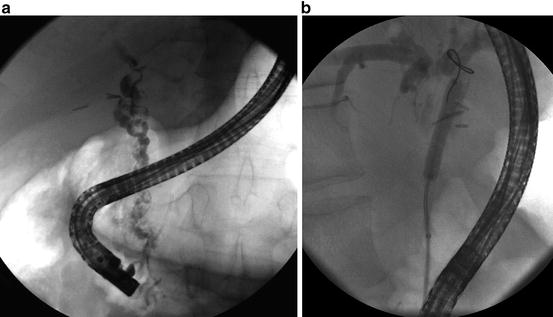

Fig. 12.1
Endoscopic management of choledocholithiasis. (a) Extensive choledocholithiasis seen throughout the extrahepatic biliary tree, particularly the common bile duct (gallbladder is surgically absent). (b) Removal of stones by endoscopic sphincterotomy followed by stone extraction with an occlusion balloon (as shown)
An alternative to biliary sphincterotomy is balloon dilation of the papilla (i.e., balloon sphincteroplasty) using small-diameter balloons (4–8 mm) [32]. This technique was introduced to preserve sphincter of Oddi anatomy and function, particularly in young patients. The majority of published literature on balloon sphincteroplasty originates from outside the USA. Two meta-analyses of randomized trials of balloon sphincteroplasty versus ES have shown that the incidence of pancreatitis and need for mechanical lithotripsy are significantly higher while the incidence of bleeding is significantly lower with balloon sphincteroplasty compared to standard ES [33, 34]. The only randomized clinical trial in the USA comparing balloon sphincteroplasty to ES was terminated early because of two deaths in young patients from post-ERC pancreatitis (PEP) after balloon sphincteroplasty, and thus the use of endoscopic balloon sphincteroplasty alone to extract stones as an alternative to ES has fallen out of favor in the USA [35, 36]. Nevertheless, sphincteroplasty still remains a viable option in patients with coagulopathy, persons with underlying portal hypertension (particularly Child’s class C33), and those with altered anatomy (e.g., Billroth II gastrojejunostomy), wherein ES is technically difficult [33]. The use of prophylactic pancreatic duct stents and/or nonsteroidal anti-inflammatory drugs (NSAIDs) in these patients should be considered to prevent PEP when sphincteroplasty alone is used to remove stones.
Removal of large bile duct stones (generally defined as ≥1.5 cm in diameter) may require additional techniques such as lithotripsy in order to be removed successfully. One form of lithotripsy is mechanical lithotripsy, in which the stone is captured in a specialized large basket and crushed [37]; the fragments are then removed using standard extraction techniques. Another form of lithotripsy is intraductal lithotripsy, which is performed by passing laser or electrohydraulic catheters into the bile duct and fragmenting stones under direct endoscopic visualization via transpapillary choledochoscope [38]. Direct visualization is essential during intraductal lithotripsy to ensure that the lithotripsy device is directed at the stone and not the bile duct wall so as to prevent biliary epithelial or transmural injury. Recently, the combination of biliary sphincterotomy and large-diameter (>12 mm and up to 20 mm) balloon dilation has been used to remove large stones and avoid the need for mechanical lithotripsy. This combination enlarges the lumen of the distal common bile duct and sphincterotomy opening, which represent the major limitations to successful extraction of large bile duct stones, and appears to be safe and is not associated with an increased risk of PEP.
When large stones cannot be removed, a biliary stent is placed to relieve biliary obstruction [39]; additional procedures can then be undertaken to remove residual stone burden.
Benign Biliary Strictures
Benign biliary strictures (BBSs) can occur due to a variety of causes (Table 12.3), although regardless of cause, endoscopic therapy generally consists of balloon dilation and placement of plastic biliary stents (Fig. 12.2) [40]. There is now substantial evidence that placement of multiple plastic stents side by side for a total of 6–12 months optimizes stricture resolution rates (>90 %) (Fig. 12.3) and minimizes recurrence as compared to placement of only one or two stents [41–44].
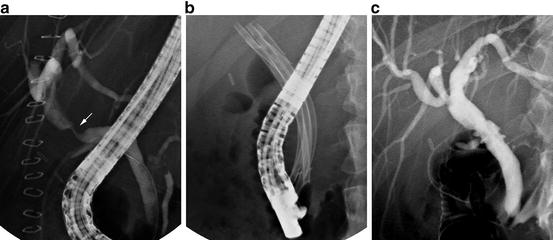
Table 12.3
Principal causes of benign biliary strictures
Operative injury |
Cholecystectomy |
Resection of choledochal cyst or other hepatobiliary lesion |
Liver transplantation (i.e., anastomotic biliary stricture) |
Antrectomy |
Pancreatitis |
Ischemia (e.g., post-liver transplantation non-anastomotic strictures) |
Portal biliopathy |
Immunoglobulin G4-related cholangiopathy |
Acquired immune deficiency syndrome (AIDS) cholangiopathy |
Eosinophilic cholangitis |
Idiopathic |

Fig. 12.2
Representative illustration of endoscopic stenting of (malignant) distal common bile duct stricture

Fig. 12.3
Endoscopic management of benign, post-liver transplantation anastomotic biliary stricture (ABS). (a) Post-liver transplantation ABS (arrow) evident on ERC. (b) Endoscopic balloon dilation followed by placement of multiple plastic biliary stents. (c) ABS resolution
More recently, the use of 8–10 mm diameter fully covered self-expandable metal stents (SEMS), which are several times the diameter of individual plastic biliary stents, has emerged as a promising alternative in the treatment of non-hilar BBS (i.e., those involving the ductal confluence); however, these stents are costly and not yet approved in the USA for BBSs [45]. Chronic pancreatitis can result in formation of distal common bile duct strictures that are commonly refractory to endoscopic therapy with plastic stents, particularly in patients with calcific chronic pancreatitis [46]. In these cases, maximal multiple plastic stent therapy, or alternatively fully covered SEMS, can be attempted [47].
Biliary Strictures in LT Patients
As with other benign biliary strictures, ERC is indicated for treatment of post-LT biliary strictures [44, 48]. Placement of multiple (up to 9 [44]) plastic biliary stents for a total of at least 12 months has been shown to optimize stricture resolution rates (Fig. 12.3) [44, 46, 48]. While the response to this multiple or “maximal” endoscopic stenting protocol is favorable for most BBSs (Fig. 12.4) [44, 49], therapeutic outcomes depend at least in part on the underlying etiology (e.g., non-anastomotic post-LT strictures are generally more challenging to treat) [43, 44, 50], time since LT [51], immunosuppressive regimen (sirolimus being a risk factor for adverse ERC outcomes) [52, 53], and endoscopist experience [54].
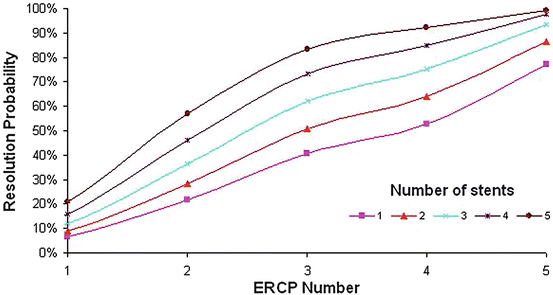

Fig. 12.4
Cox proportional hazard model demonstrating ABS resolution probability as a function of number of stents at each ERCP. Adapted from Tabibian JH, Asham EH, Han S, et al. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video). Gastrointest Endosc 2010;71:505–12. With permission from Elsevier Limited
Malignant Biliary Obstruction
Endoscopic relief of malignant biliary obstruction is achieved by placement of one (distal) or more (hilar) large-bore (10 Fr) plastic stents or SEMS across the malignant stricture(s). The endoscopic approach depends on whether the obstructive is distal to the bifurcation of the common hepatic duct or involving the bifurcation (i.e., hilar obstruction).
Malignant Distal (Non-hilar) Biliary Obstruction
Distal biliary obstruction due to malignancy (Fig. 12.5), regardless of etiology (e.g., CCA, pancreatic adenocarcinoma), can be effectively treated using a single biliary stent. ERC with biliary stent placement is now accepted as a viable alternative to palliative surgical bypass and can be performed safely in an outpatient setting [55]. Relief of obstruction is as effective as with surgical biliary bypass, but with lower morbidity and mortality, albeit with an increased re-obstruction rate [56].
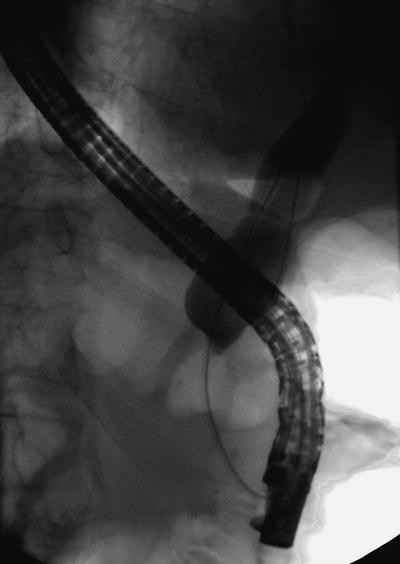

Fig. 12.5
Long distal common bile duct stricture with bird’s beak appearance, highly suggestive of underlying malignancy
The most common cause of distal bile duct obstruction is adenocarcinoma of the pancreatic head. The role of endoscopic biliary decompression in patients with distal bile duct obstruction due to pancreatic adenocarcinoma depends heavily on the clinical scenario, and in fact routine preoperative ERC for biliary decompression is discouraged in patients with surgically resectable disease. Several studies have shown that preoperative endoscopic stent placement does not improve surgical outcome and in fact increases overall morbidity and adverse events from ERC that may result in delay of or even prohibit surgical resection [57, 58]. ERC is indicated, however, in cases complicated by acute cholangitis and severe pruritus [59]. ERC is also indicated when neoadjuvant chemoradiation is planned considering the prolonged period of time to surgical resection. In such cases, the use of a short-length (≤6 cm) SEMS (covered or uncovered) (Fig. 12.6) appears to be the best option based on data showing a higher rate of stent occlusion in patients receiving plastic biliary stents as compared to a SEMS [60, 61].
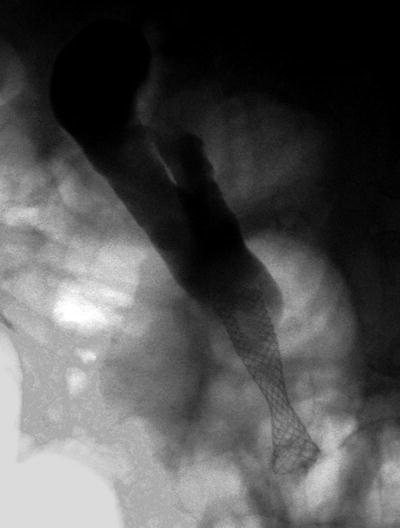

Fig. 12.6
Biliary self-expanding metal stent (SEMS) placed for palliation of a malignant distal biliary stricture
The main limitation to plastic stent placement is stent occlusion as a result of bacterial biofilm formation or reflux of food matter and as a function of time since stent placement [44, 62]; therefore, in the comparative trials of endoscopy versus surgery, the shorter length of initial hospital stay in the endoscopy group was offset by the need for subsequent hospitalizations and repeat ERC to manage plastic stent occlusion. The use of SEMS has largely overcome the issue of bacterial biofilm formation, and randomized controlled trials have shown superior patency rates for uncovered biliary SEMS when compared to plastic stents [42, 63]. It is notable to recognize that the cost of a SEMS is substantially greater than that of a plastic stent; however, placement of a SEMS can be cost effective as long as projected life expectancy is longer than 3–6 months; thus, this should be taken into account when deciding between plastic and metal biliary stents [64].
Other factors to be considered in deciding between a plastic stent and a SEMS include a patient’s adherence and ability to return for care, including management of stent occlusion. Uncovered SEMS occlusion is generally managed with placement of a plastic stent or a new SEMS within the existing one. Covered SEMS have been introduced more recently in an attempt to reduce tumor ingrowth (Fig. 12.7) and tissue hyperplasia through stent interstices and thereby potentially extending patency; although there may be less stent occlusion with covered SEMS, this appears to come with a greater cost, higher migration rates [65], and possibly an increased risk of acute cholecystitis [66, 67]. Advantages of covered SEMS are ease of removability and revision compared to uncovered SEMS, which can become imbedded into the surrounding bile duct. Removability may be particularly important in patients who are later found to have benign disease (e.g., autoimmune pancreatitis) or curable malignancy (e.g., lymphoma [68]).
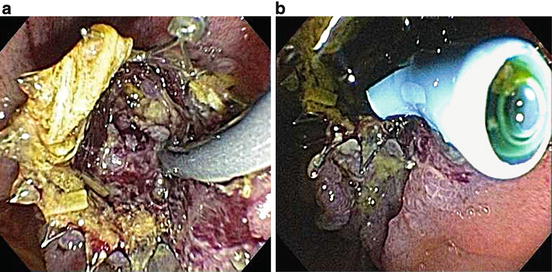

Fig. 12.7
Tumor ingrowth. (a) Ingrowth of tumor and debris is seen obstructing the lumen of a SEMS. (b) Palliation is provided by placement of a plastic biliary stent through the SEMS
Malignant Hilar Biliary Obstruction
Hilar biliary strictures may be caused by CCA (i.e., Klatskin tumor) or metastatic disease. The approach to biliary obstruction involving the hepatic hilum is endoscopically more challenging than that of distal biliary obstruction [69], with procedural and clinical success rates depending on biliary tract anatomy and endoscopist experience. Success rates also depend on the need for unilateral versus bilateral biliary stent placement, which is a function of potential for resectability, Bismuth classification, presence and location of liver atrophy, intrahepatic tumor burden, and presence of PSC [70].
Most patients with hilar malignant biliary obstruction will be adequately palliated with unilateral biliary stenting and drainage, i.e., with only one side being accessed and therefore endoscopically contaminated [71]; patients who have had both the left and right biliary systems accessed and injected with contrast require stenting of both to reduce the risk of acute cholangitis [72]. To avoid such contrast injection in cases where it is not necessary, guidewires are passed into intrahepatic ducts without injecting contrast using an imaging-guided (MRI, computed tomography [CT]) approach. Pre-ERC abdominal imaging may reveal atrophy of one lobe of the liver and should be specifically avoided during ERC since contamination will require drainage to prevent cholangitis but will likely not facilitate palliation.
Unlike patients with malignant distal biliary obstruction, the advantages of SEMS placement are not as clear-cut in patients with hilar disease, and re-intervention for management of stent occlusion can be particularly difficult when bilateral biliary stents have been placed [73]. In a prospective single-arm pilot study of SEMS in 17 patients with Bismuth type II and III hilar CCA, median stent patency was 12 months [74]. Similarly, in a non-comparative single-arm study, insertion of a Wallstent (a type of SEMS) was found to be safe and feasible and resulted in successful palliation without the need for further biliary intervention in 69 % of patients with nonresectable hilar CCA [75]. In a more recent prospective observational cohort study of patients with hilar tumors treated with plastic or metal stents, patients receiving metal stents had significantly lower rates of post-procedural complications and need for percutaneous drainage [76]. These encouraging results suggest that SEMS may offer similar benefits for malignant hilar biliary obstruction as they do for distal biliary obstruction.
To summarize regarding the role of endoscopic stenting in malignant hilar biliary obstruction, patients with unresectable hilar CCA who undergo ERC for palliation should receive unilateral stenting with unilateral contrast injection. One biliary stent, be it plastic or metal, is generally adequate to achieve palliation, although treatment may need to be individualized (e.g., extensive, bilateral stenting in some cases) [77]. SEMS appear to offer more durable palliation than plastic stents but are only cost effective when expected survival is at least 3 months, as mentioned above [78, 79]. Lastly, from an endoscopic perspective, achieving successful drainage is more difficult technically for hilar as compared to distal biliary obstruction.
For patients in whom jaundice does not resolve after endoscopic placement of plastic stents, several other endoscopically administered modalities can be used for palliation of unresectable hilar CCA [80]. Photodynamic therapy (PDT) is associated with significant improvements in cholestasis, quality of life, and survival (as compared with historical controls) and can be maintained for an extended period [81, 82]. However, the initial studies on PDT for hilar CCA were performed outside the USA, in countries where smaller, more flexible laser fibers are available. In the USA, biliary PDT is currently performed utilizing rigid laser fibers which are used for the treatment of neoplastic esophageal diseases. Nevertheless, PDT trials in the USA have shown promising results [83, 84] among select centers with expertise in this procedure.
More recently, intraductal radiofrequency ablation has been used to treat CCA [85]. The radiofrequency ablation probe is 10 Fr in diameter and flexible and can be used with standard electrosurgical generators. Preliminary data are promising, and indeed this technology may ultimately replace biliary PDT. Endoscopically delivered brachytherapy, either low dose or high dose, is also available, but this therapy is generally reserved for patients who are part of an LT protocol [86].
Primary Sclerosing Cholangitis with Indeterminate Dominant Strictures
In addition to the indications for diagnostic ERC in patients with PSC described above, patients with PSC may benefit from therapeutic ERC for several indications, including treatment of a dominant stricture or biliary lithiasis [87]. Patients with a dominant stricture usually present with progressive biliary obstruction and associated signs (e.g., rising serum alkaline phosphatase and/or bilirubin, acute cholangitis) and symptoms (e.g., pruritus, choluria). Endoscopic treatment of a dominant stricture involves balloon dilation, often in combination with short-term (<8 weeks) placement of a large-bore (10 Fr) stent. Success rates, with clinical, biochemical, and radiologic improvement as endpoints, have ranged from 65 to 100 % with this approach, although currently available endoscopic interventions are not thought to impact the long-term outcomes (i.e., CCA, need for LT, or death) of PSC [4, 88].
In addition to relieving biliary obstruction during ERC, attempts to exclude CCA should also be undertaken. Routine brush cytology has a low sensitivity in the patients with PSC, but the diagnostic yield can be augmented by also performing fluorescence in situ hybridization (FISH) on biliary brush specimens [8, 89]. Cholangioscopy may further improve detection of malignancy in these patients (Fig. 12.8), but published studies are limited, and reliability appears to be less in patients with PSC than in those with de novo strictures due to difficulty in distinguishing benign from malignant strictures [90]. More recently, probe-based confocal laser endomicroscopy has been used [23]. This technique requires pre-procedural injection of fluorescein contrast and use of a specialized probe that allows real-time visualization of cellularity [91]. More data are needed before this technique can be recommended routinely.
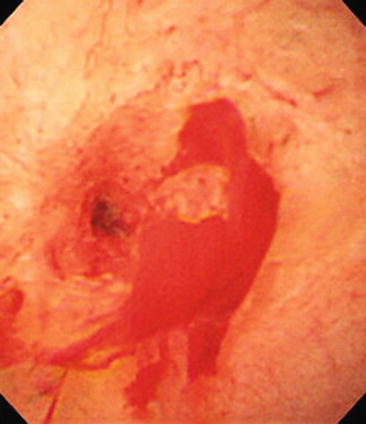

Fig. 12.8
Cholangioscopic image demonstrating tortuous blood vessels and biliary mucosal friability. A diagnosis of cholangiocarcinoma was ultimately made by a combination of diagnostic techniques
Diagnostic and Therapeutic Biliary Endoscopy in Patients with Altered Bilio-Enteric Anatomy
ERC was once considered impossible in patients with surgically altered anatomy, and instead percutaneous or surgical approaches were historically used. With increasing experience, however, initially using colonoscopies [92], and in the advent of balloon enteroscopy [54, 93], biliary endotherapy can now be achieved in patients with surgically altered anatomy, including patients with Roux-en-Y anastomoses and post-Whipple anatomy (Fig. 12.9). The most difficult anatomy is that following Roux-en-Y gastric bypass, in these patients, options include unique endoscopic approaches [94] or a combined endoscopic and laparoscopic assisted approach whereby access to the excluded stomach and antegrade passage of a standard side-viewing duodenoscope into the duodenum are possible [95]. Furthermore, percutaneous-endoscopic hybrid access approaches have been recently described to treat biliary diseases; “percutaneous assisted transluminal endoscopic therapy” (PATENT) [96] has been used to treat gallbladder disease and to access the excluded stomach in order to pass antegrade duodenoscopes in patients with RYGB anatomy. Using this approach, management of CBD stones and other obstructive biliary processes can be achieved entirely endoscopically [97].