This review discusses the indications, technical aspects, and comparative effectiveness of the endoscopic treatment of upper gastrointestinal bleeding caused by peptic ulcer. Pre-endoscopic considerations, such as the use of prokinetics and timing of endoscopy, are reviewed. In addition, this article examines aspects of postendoscopic care such as the effectiveness, dosing, and duration of postendoscopic proton-pump inhibitors, Helicobacter pylori testing, and benefits of treatment in terms of preventing rebleeding; and the use of nonsteroidal anti-inflammatory drugs, antiplatelet agents, and oral anticoagulants, including direct thrombin and Xa inhibitors, following acute peptic ulcer bleeding.
Key points
- •
Endoscopy should be performed within 24 hours of presentation in patients with upper gastrointestinal bleeding.
- •
Pre-endoscopic prokinetics should be considered in patients who are suspected of having a significant amount of blood in the upper gastrointestinal tract, such as those with positive nasogastric aspirate or active hematemesis, to improve the endoscopic view and decrease the need for repeat endoscopy.
- •
Endoscopic therapy should be performed in lesions with high-risk stigmata (Forrest classification I–IIB) with combination therapy or monotherapy using current endoscopic hemostatic agents except for epinephrine, which should not be used alone as definitive therapy.
- •
Postendoscopic care includes testing and eradication of Helicobacter pylori , avoidance of nonsteroidal anti-inflammatory drugs whenever possible, and the use of proper gastroprotective strategies in patients requiring ongoing antiplatelet and anticoagulant therapy.
Introduction
Peptic ulcers are the most frequently encountered cause of upper gastrointestinal bleeding (UGIB), with an annual incidence of 19.4 to 57.0 per 100,000 individuals. Ulcers account for one-third to half of all presentations of acute UGIB. A large United States inpatient database reveals declining trends in the incidence of both UGIB and peptic ulcer bleeding. Similarly, the mortality from UGIB has also decreased from 2.95% to 2.45%. In the management of UGIB, the use of endoscopy plays a fundamental role in the diagnosis, treatment, and prognostication of UGIB. Pre-endoscopic considerations such as the use of prokinetics and timing of endoscopy are reviewed, with a focus on the endoscopic management of peptic ulcer bleeding. Such management includes the use of the available hemostatic modalities, including emerging therapies, which will be reviewed along with their comparative effectiveness. Proton-pump inhibitors (PPIs) after endoscopy, indications for second-look endoscopy, and the use of secondary pharmacologic prophylaxis are also discussed. Issues of risk stratification and initial resuscitation are discussed in an article elsewhere in this issue by Meltzer and Klein.
Introduction
Peptic ulcers are the most frequently encountered cause of upper gastrointestinal bleeding (UGIB), with an annual incidence of 19.4 to 57.0 per 100,000 individuals. Ulcers account for one-third to half of all presentations of acute UGIB. A large United States inpatient database reveals declining trends in the incidence of both UGIB and peptic ulcer bleeding. Similarly, the mortality from UGIB has also decreased from 2.95% to 2.45%. In the management of UGIB, the use of endoscopy plays a fundamental role in the diagnosis, treatment, and prognostication of UGIB. Pre-endoscopic considerations such as the use of prokinetics and timing of endoscopy are reviewed, with a focus on the endoscopic management of peptic ulcer bleeding. Such management includes the use of the available hemostatic modalities, including emerging therapies, which will be reviewed along with their comparative effectiveness. Proton-pump inhibitors (PPIs) after endoscopy, indications for second-look endoscopy, and the use of secondary pharmacologic prophylaxis are also discussed. Issues of risk stratification and initial resuscitation are discussed in an article elsewhere in this issue by Meltzer and Klein.
Prokinetics
Prokinetic agents such as erythromycin and metoclopramide can be administered before endoscopy to improve endoscopic yield and reduce the need for a repeat endoscopy. Erythromycin, a motilin agonist, can be given at a dose of 250 mg intravenously, and metoclopramide 10 mg intravenously 30 to 60 minutes before endoscopy. The use of erythromycin is favored based on current data. Doses used in the literature range from 3 to 4 mg/kg of erythromycin administered 20 to 90 minutes before endoscopy. Furthermore, the QT-interval–prolonging effect of erythromycin should be taken into consideration, and an electrocardiogram first performed.
The use of prokinetics should be considered in acute UGIB, particularly when targeting patients with active bleeding and/or evidence of blood in the stomach. A meta-analysis comprising 3 randomized controlled trials (RCTs) with erythromycin and 2 abstracts with metoclopramide included a total of 162 patients, all showing evidence of active bleeding with blood in the stomach, showed that a prokinetic agent, in comparison with placebo or no treatment, led to a significant reduction in the need for repeat endoscopy (odds ratio [OR] 0.55; 95% confidence interval [CI] 0.32–0.94). This effect was not preserved when analyzing metoclopramide alone (OR 1.22; 95% CI 0.35–4.25). No differences were noted for blood transfusions or need for surgery, while mortality was not analyzed. A more recent meta-analysis solely looking at erythromycin showed similar results, with improvement in the visualization of the gastric mucosa (OR 3.43; 95% CI 1.81–6.50), and a decrease in the need for a second-look endoscopy (OR 0.47; 95% CI 0.26–0.83). Of note, the effect of erythromycin in decreasing units of blood transfused and length of stay in hospital reached significance when an additional trial that only included patients with variceal bleeding was added to the meta-analysis.
Pre-endoscopic intravenous PPI administration and nasogastric lavage can also be considered before endoscopy; these details are discussed elsewhere.
Timing of endoscopy
The performance of early endoscopy within 24 hours of patient presentation is warranted for most patients. This practice has been shown to be safe for all patients at risk, allows for earlier discharge of low-risk patients, and improves outcomes in those at high risk. Lower costs are also associated with early discharge after endoscopy of low-risk patients.
Several RCTs and retrospective cohort studies have examined very early endoscopy at less than 2 to 3, less than 6, less than 8, and less than 12 hours, compared with less than 24 to 48 hours. Faster time to endoscopy yielded higher rates of finding high-risk stigmata of bleeding at endoscopy, and similarly higher rates of hemostasis being performed without demonstrable effects on outcomes of rebleeding, need for surgery, or duration of hospitalization. This finding contrasts with those of studies looking at early endoscopy (<24 hours) showing a significant decreased length of stay in hospital. Moreover, decreased rebleeding and need for surgery were also observed, particularly among high-risk patients (ulcer with active bleeding or visible vessel, or varices).
In higher-risk patients, one study randomizing patients to endoscopy at less than 12 hours versus greater than 12 hours from patient presentation detected a significantly reduced need for blood transfusions, and shorter hospitalization (4 vs 14.5 days) in the subgroup of patients with coffee grounds or bloody nasogastric aspirate. In parallel, one observational study demonstrated, using a receiver-operating curve analysis, that a longer time to endoscopy (>13 hours) predicted all-cause in-hospital mortality in patients with a Blatchford score of 12 or higher. The robustness of the results is limited by the lack of randomization and adjustment for possible confounders that can influence mortality. As a result, the American College of Gastroenterology guidelines on ulcer bleeding suggest that endoscopy within 12 hours may be considered in high-risk patients. Similarly, the United Kingdom UGIB toolkit produced by the Academy of Royal Medical Colleges recommends endoscopy at less than 6 to 12 hours in “urgent-risk” patients, a practice that is currently not supported by high-quality evidence for nonvariceal UGIB (NVUGIB), though appropriate in the context of suspected variceal bleeding, in which case endoscopy within 12 hours is suggested.
Data from bleeding registries show that a significant proportion of patients have a delay greater than 24 hours before undergoing upper endoscopy. Though variable, rates of early endoscopy range from 50% in a United Kingdom bleeding audit up to 82% in a large Danish registry. Reasons behind such delays are likely multifactorial; however, several reports from administrative databases relay a “weekend effect” whereby patients presenting on weekends are less likely to undergo early endoscopy, and have higher mortality, which may or may not result from longer endoscopy delays. Nevertheless, endoscopy within 24 hours of patient presentation should be targeted as a stand-alone quality indicator when managing patients with acute UGIB.
Endoscopic diagnosis and risk stratification
The endoscopic findings of a bleeding ulcer have prognostic implications in terms of rebleeding, need for surgery, and mortality. As a result, they are integrated in risk assessment scores such as the complete Rockall score, and are further used to determine the need for endoscopic hemostasis. The stigmata of recent bleeding are used to characterize the endoscopic appearance at the base of the bleeding ulcer, and are commonly categorized according to the Forrest classification into high-risk stigmata (HRS) and low-risk stigmata (LRS) ( Table 1 ). HRS comprise active spurting bleeding (Forrest IA), active oozing bleeding (Forrest IB), nonbleeding visible vessel (Forrest IIA), and adherent clot (Forrest IIB). Low-risk lesions that include flat pigmented spots (Forrest IIC) and clean base ulcers (Forrest III) are more prevalent, reaching a prevalence of more than 50%. Older data predating the routine use of endoscopic hemostasis reveal a natural history with rebleeding rates of 55% (17%–100%) (Forrest IA and IB), 43% (0%–81%) (Forrest IIA), 22% (14%–36%) (Forrest IIB), 10% (0%–13%) (Forrest IIC), and 5% (0%–10%) (Forrest III). Significant interobserver variability has been reported in the identification of endoscopic stigmata. Nonetheless, when ulcer bleeding stigmata are surveyed with sequential endoscopic evaluations, an evolution of stigmata is noted, suggesting various phases of healing whereby a bleeding vessel progressively evolves after endoscopic therapy into a clean base ulcer. Furthermore, most rebleeding episodes were noted within the first 72 hours, consistent with the natural history of ulcer healing.
| Stigmata of Recent Hemorrhage | Forrest Classification | |
|---|---|---|
| Active spurting bleeding | High-risk stigmata | IA |
| Active oozing bleeding | IB | |
| Nonbleeding visible vessel | IIA | |
| Adherent clot | IIB | |
| Flat pigmented spot | Low-risk stigmata | IIC |
| Clean base | III |
In patients with LRS ulcers, endoscopic hemostasis has not been shown to alter outcomes. The treatment of patients with pigmented spots or clean base ulcers therefore consists of oral acid suppressive pharmacotherapy alone. In contradistinction, endoscopic therapy in ulcer bleeding has been well established to decrease continued or recurrent bleeding, surgery, and mortality, particularly in patients with active bleeding and nonbleeding visible vessel. For such patients, the magnitude of improvement in outcome measures from an earlier meta-analysis of 30 RCTs is as follows: further bleeding (OR 0.38; 95% CI 0.32–0.45), surgery (OR 0.36; 95% CI 0.28–0.45), and mortality (OR 0.55; 95% CI 0.40–0.6). The benefits of endoscopic therapy for patients with HRS were confirmed in a more recent meta-analysis demonstrating the superiority of any endoscopic method over pharmacotherapy (which included one trial using high-dose intravenous PPI) for rebleeding (OR 0.35; 95% CI 0.27–0.46), surgery (OR 0.57; 95% CI 0.41–0.81), and mortality (OR 0.57; 95% CI 0.37–0.89). Similarly, Laine and McQuaid found decreased rebleeding and surgery with endoscopic therapy in comparison with no endoscopic therapy for active bleeding and nonbleeding visible vessel. However, they did not detect statistically significant benefits with regard to mortality, or in any outcomes among patients with adherent clots. Endoscopic hemostasis is therefore indicated in all patients with HRS, whereas ulcers with LRS can be managed with pharmacotherapy only, and do not warrant therapy. The more detailed management of lesions with adherent clots is somewhat controversial (see later discussion).
Endoscopic therapy
Endoscopic therapy is indicated in patients presenting with NVUGIB and HRS on endoscopy (see Table 1 ). Meta-analyses have found that endoscopic therapy for ulcers with these features significantly decreased rebleeding, surgery, and mortality. In contradistinction, low-risk lesions such as ulcers with flat, pigmented spot (Forrest IIC) or clean based (Forrest III) are associated with lower incidences of rebleeding, and endoscopic therapy has not been shown to be beneficial. In terms of endoscopic modalities, there are several techniques that have been developed including injection, thermal, and mechanical therapies and, more recently, hemostatic powders. In addition, several ancillary methods to improve endoscopic risk stratification of bleeding lesions and hemostasis such as Doppler ultrasonography, magnification endoscopy, and chromoendoscopy, have also emerged. The following is an overview of the different endoscopic therapies, with a specific focus on the techniques involved in their successful deployment.
Injection Therapy
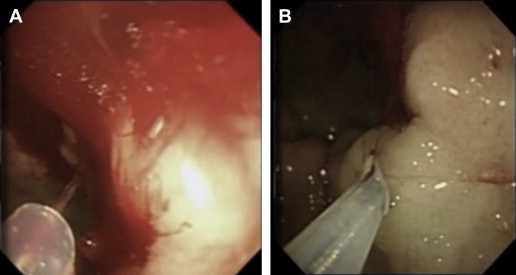
Different injectates have been developed, including epinephrine, hypertonic saline, sclerosants (polidocanol, ethanolamine, absolute alcohol, sodium tetradecyl sulfate), and tissue adhesives (cyanoacrylate, thrombin, fibrin). Injection therapy has been widely performed most likely because of its ease of use, availability, and extensive experience with its application by most endoscopists. Injections are delivered through a 25-gauge retractable catheter ( Fig. 1 A). Epinephrine (1:10,000 or 1:20,000 dilution) is the most common injectate used in the control of NVUGIB, and should be performed in increments of 0.5 to 1.5 mL in all 4 quadrants around the ulcer base with or without injections into the center of the ulcerated lesion itself (see Fig. 1 B). It is important that the main mechanism responsible for successful hemostasis is likely due to the tamponade effect from the volume of the injection rather than the vasoconstrictive mechanism of the epinephrine or resulting platelet aggregation. In fact, randomized controlled data suggest that higher total volumes (13–45 mL) per bleeding lesion may decrease rebleeding rates, presumably because of the greater tamponade effect. The application of epinephrine is simple and requires little skill, such that its application can be easily performed with both tangential and en-face positioning, and may be the initial agent of choice in massive gastrointestinal (GI) hemorrhage where rapid control of the bleeding field with other modalities can be difficult to achieve because of the obscured view. However injection therapies have been shown to be inferior to monotherapies with both thermal or mechanical modalities and combination therapies. Pooled data for injection therapy (including trials on epinephrine monotherapy, alcohol monotherapy, and a combination of other injectates) reveals a reduction in rebleeding (OR 0.43; 95% CI 0.24–0.78) compared with pharmacotherapy in patients with HRS. Despite evidence of comparable initial hemostasis success, epinephrine injection is inferior to other monotherapies (thermal, fibrin glue, and clip), as already discussed, with a higher incidence of rebleeding (OR 1.72; 95% CI 1.08–2.78) and surgery (OR 2.27; 95% CI 1.02–5.00) when used alone. In a meta-analysis of 16 RCTs, Calvet and colleagues demonstrated that epinephrine injection, followed by a second modality, provides additional benefits with regard to reducing rebleeding (OR 0.53; 95% CI 0.40–0.69), surgery (OR 0.64; 95% CI 0.46–0.90), and mortality (OR 0.51; 95% CI 0.31–0.84) when compared with epinephrine monotherapy. Mortality benefits were not reproduced in more recent analyses. In summary, there is strong evidence suggesting that epinephrine injection should not be used alone for the treatment of ulcer bleeding with HRS.
The role of other injectates, such as sclerosants and tissue adhesives, in the management of NVUGIB are less well defined. Sclerosants including polidocanol, ethanolamine, absolute alcohol, and sodium tetradecyl sulfate are associated with a risk of tissue necrosis, perforation, and vessel thrombosis. Sclerosant therapy has nonetheless been associated with significant benefits in reducing rebleeding, surgery, and mortality in comparison with no therapy, and can be considered in the management algorithm of NVUGIB. Tissue adhesives such as cyanoacrylate, thrombin, and fibrin, on the other hand, have not been evaluated as extensively, and are often limited by their high acquisition costs and lack of availability. In comparison with epinephrine, these agents are rarely used in the routine treatment of patients with NVUGIB.
Thermal Therapy
Thermal therapy applies heat or electric current to bleeding lesions, which can lead to coagulation of vessels and successful hemostasis. Thermal treatment can be divided into contact (electrocoagulation or heater probe) and noncontact techniques.
Contact electrocoagulation
Endoscopic contact electrocoagulation is available as monopolar, bipolar (BEC), or multipolar (MEC) electrocautery. Unlike the monopolar modality, the electric circuit with BEC or MEC terminates locally at the tip of the probe and decreases in intensity as the target tissue desiccates with electrocautery, thereby limiting the depth of penetration and decreasing the risk for perforation. Optimal BEC/MEC is best performed using a large-diameter (10F, 3.2 mm) probe with firm, constant pressure on the high-risk lesion and application of low energy (15 W) electrocoagulation for 10 to 12 seconds until flattening of vessels or adequate coagulation of the stigmata ( Fig. 2 ). In other words, the goal is to achieve coaptive coagulation, with hemostasis occurring from physical occlusion and tamponade of the vessels in the bed of the bleeding lesion, followed by thermal coagulation. Data from porcine models suggest that longer duration of electrocoagulation (tamponade station) increases the energy and coagulation, whereas increasing the watt setting does not improve the transfer of coagulation owing to the rapid increase in impedance. Therefore, long-duration (10–12 seconds) and low-energy (15 W) electrocoagulation are preferred for optimal coaptive coagulation. In terms of delivery, contact electrocoagulation is easy to use, allows for simultaneous, foot pedal–controlled water irrigation, and is effective in both bleeding and nonbleeding HRS. However, MEC/BEC may be difficult to use in the tangential position given that electric current occurs at the tip of the catheter.
Contact heater probe
The heater probe dissipates heat at the tip and sides of the probe, leading to coagulation of high-risk lesions in both the en-face and tangential position. Similarly to contact electrocoagulation, the application is best performed using a large-diameter probe (10F, 3.2 mm) and requires firm constant pressure with the target lesion; however, unlike BEC or MEC, coagulation is provided in the form of heat energy (25–30 J) and is delivered in a pulsatile fashion (4–5 pulses). The main advantage of the heater or gold probe over contact electrocoagulation is its ability to provide coagulation through the tip and sides of the probe, therefore allowing for ease of application in both the tangential and en-face position. The heater probe also allows for foot pedal–controlled water irrigation, and is effective in both bleeding and nonbleeding HRS.
The use of contact thermal therapy, namely heater probe and electrocoagulation, is beneficial in achieving hemostasis in patients with high-risk lesions, as evidenced by reduced rebleeding (OR 0.44; 95% CI 0.36–0.54), surgery (OR 0.39; 95% CI 0.27–0.55), and mortality rates (OR 0.58; 95% CI 0.34–0.98) when compared with no endoscopic treatment, although the impact on mortality was not reproduced in a separate meta-analysis (OR 0.64; 95% CI 0.25–1.65). Aside from the previously discussed benefits over epinephrine monotherapy, comparison of contact thermal therapy with other modalities, alone or in combination, have not yielded consistent results to favor the use of one approach over another. It is worth mentioning that although pooled meta-analytical data comparing the combination of injection and thermal therapy with thermal monotherapy did not show any significant differences in rebleeding, subgroup analysis favored combination therapy after the removal of 2 less generalizable trials, resulting in decreased risks of rebleeding (OR 0.37; 95% CI 0.14–0.97). Overall, as data to confidently support the use of one modality over another are lacking, technical considerations may better dictate the use of thermal therapy. In addition, thermocoagulation can be used either alone or in combination with injection therapy.
Noncontact thermal therapy
Argon plasma coagulation (APC) is unique in its ability to deliver electrocautery in a noncontact fashion through the delivery of monopolar, alternating current through ionized argon gas. Electrical current is delivered to the target tissue via electrically charged argon gas that is propelled from the catheter tip. Coagulation is mostly superficial (up to 2.4 mm according to in vitro studies ), given that the argon plasma beam shifts away from coagulated tissue, which loses its conduction ability because of increases in electrical resistance with desiccation, therefore preventing further tissue damage and perforation. In terms of application, the optimal distance between probe and target tissue is estimated to be 2 to 8 mm. Direct contact between the probe and the target lesion should be avoided to prevent submucosal dissection and submucosal argon gas insufflation, which causes significant pain, pneumatosis, and risk of perforation. APC seems to be ideal for shallow and broadly defined bleeding lesions such as angiodysplasia, radiation telangiectasia, and gastric antral vascular ectasia, also known as watermelon stomach.
The use of APC in ulcer bleeding has been evaluated in several trials that compared this approach with a variety of other modalities including heater probe, sclerotherapy, epinephrine in combination with heater probe, endoclips, or polidocanol, with no differences in patient outcomes. However, decreased rebleeding was seen when compared with distilled water injection. A single small randomized trial using epinephrine plus APC versus heater probe in patients with high-risk ulcer bleeding showed improved initial hemostasis in the APC arm.
Endoclips
A wide variety of hemostatic mechanical therapies have been developed over the years; however, the endoscopic deployment of endoclips remains the most extensively studied and widely available technique in the management of NVUGIB. It achieves hemostasis through direct compression or tamponade of vessels and tissue approximation of bleeding stigmata ( Fig. 3 ). Moreover, unlike most other endoscopic devices, the use of endoclips is usually associated with minimal to no tissue damage, therefore leading potentially to faster ulcer healing. However, its deployment requires precision, en-face positioning, and may be inadequate in fibrotic ulcer beds given its weak tensile strength. It is also noteworthy that endoclips come in many different sizes, lengths, and shapes, grasping and rotational abilities, and deployment mechanisms. Therefore, familiarity with locally available endoclips should be acquired before their use in emergency situations.
The use of endoclips is effective for ulcer bleeding and can be used alone or in combination with epinephrine, or as an alternative to thermal coagulation. Although endoclips have not been compared with pharmacotherapy alone, several RCTs have assessed the benefit of endoclips with or without epinephrine injection, and in comparison with injection and thermal therapy. Again, benefits in rebleeding (OR 0.22; 95% CI 0.09–0.55) and need for surgery (OR 0.22; 95% CI 0.06–0.83) are significant only when endoclips are compared with epinephrine alone. A meta-analysis evaluating 15 studies including 1156 patients highlighted the important heterogeneity across trials that have compared endoclips with thermocoagulation, with or without injection. Individual trials have reported disparate results regarding the superiority or inferiority of endoclips in comparison with thermal therapy. Furthermore, one meta-analysis demonstrated a benefit of endoclips over thermal therapy for rebleeding (OR 0.24; 95% CI 0.06–0.95), though statistically less robust. Additional considerations such as ulcer location can help direct the decision to use endoclips rather than another hemostatic modality, as higher endoclip failure is seen for ulcers situated in the posterior wall of the duodenum and gastric body, and along the lesser gastric curvature.
In addition to the general recommendation to avoid epinephrine monotherapy, some organizations have recently suggested that thermal or combination therapy may be favored over endoclips or sclerosants when managing actively bleeding lesions, and that thermal therapy should be used with epinephrine based on lower-quality evidence.
Endoscopic hemostatic powders
Recently, novel endoscopic topical hemostatic powders such as the Ankaferd Blood Stopper (ABS), EndoClot, and TC-325 have been adapted to digestive endoscopy and the management of gastrointestinal bleeding (GIB). ABS (Ankaferd Health Products Ltd, Istanbul, Turkey) is a herbal extract derived from 5 different plants that achieves hemostasis by promoting the formation of a protein network behaving as an anchor for erythrocyte aggregation. This agent, however, is not available in North America.
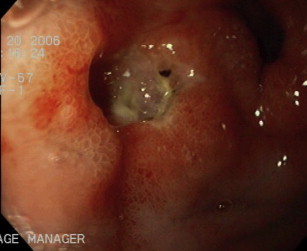
The EndoClot Polysaccharide Hemostatic System (EndoClot-PHS; EndoClot Plus, Inc, Santa Clara, CA, USA) is another emerging hemostatic powder composed of a biocompatible, nonpyogenic, starch-derived compound. It achieves hemostasis by effecting mechanical tamponade and rapid absorption of water from serum, leading to concentration of platelets and clotting factors and thereby accelerating the clotting cascade. At present, the literature on EndoClot is limited to 1 abstract reporting its successful use as an adjuvant therapy in UGIB in 6 patients. Therefore more data are needed with regard to GIB, and the EndoClot is currently not approved for use in North America. TC-325 (Hemospray; Cook Medical, Winston-Salem, NC, USA) is composed of a proprietary inorganic biologically inert powder that, when put in contact with moisture in the GI tract, becomes coherent, thus serving as a mechanical barrier for hemostasis. In addition, it may provide a scaffold, enhancing platelet aggregation and possibly activating clotting factors. However, the powder only adheres to actively bleeding lesions, so its use in high-risk lesions without active spurting or oozing is likely ineffective in providing appropriate hemostasis. The endoscopic powder is propelled from a canister under CO 2 pressure and is delivered through a catheter onto the bleeding lesion ( Fig. 4 A, B). The TC-325 catheter should be maintained 1 to 2 cm from the high-risk lesion, and application should be performed in a noncontact fashion (see Fig. 4 C). The endoscopic powder will aggregate immediately when it comes into contact with moisture; therefore, efforts should be made to keep the tip of the catheter dry and to avoid suctioning while it is in use or while the powder is settling. Flushing the accessory channel of the endoscope with a 60-mL syringe of air before TC-325 application is also recommended to ensure that the tip of the catheter does not come into contact with moisture during insertion. With the current second-generation Hemospray delivery system, application of the powder occurs by engaging the trigger button, which results in an initial burst of CO 2 gas followed by the release of the hemostatic powder. The accumulation of CO 2 gas may become uncomfortable for the patient. Advantages associated with TC-325 use include ease of application with a lack of need for precise targeting (although the powder needs to reach the actively bleeding site), noncontact and nontraumatic hemostasis, and ability to cover large surfaces of bleeding. As such it may be the ideal modality for massive NVUGIB where rapid control of the bleeding field is critical, and in hemorrhage arising from GI malignancies where bleeding often stems from friable, irregular, and diffusely bleeding surfaces.
Sung and colleagues first described the use of TC-325 in GIB in a prospective pilot study involving 20 patients with NVUGIB treated with Hemospray, resulting in an immediate hemostasis of 95%. Subsequently a multicenter, retrospective study including 63 patients with NVUGIB demonstrated a rate of immediate hemostasis and of 7-day rebleeding with TC-325 therapy for 85% (95% CI 76%–94%) and 15% (95% CI 5%–25%), respectively. More specifically, this study included 25 patients with peptic ulcer bleeding who received TC-325 monotherapy, which resulted in an immediate hemostasis of 76% (95% CI 59%–93%) and a 7-day rebleeding rate of 16% (95% CI 0%–32%). Retrospective data at a single institution recently demonstrated the versatility and effectiveness of TC-325 in both the upper and lower GI tracts in 67 cases of TC-325 therapy in patients with a variety of bleeding disorders including 21 cases of NVUGIB of nonmalignant etiology. The rate of immediate hemostasis and early rebleeding (within 72 hours of TC-325 application) for NVUGIB were 95.2% and 29.4%, respectively. In addition, the powder residency time on the bleeding lesions was demonstrated to be less than 24 hours. Therefore, the current thinking is that TC-325 should not be used as the sole modality in lesions at high risk of rebleeding following the first 24 hours, such as peptic ulcer bleeding with HRS; indeed other modalities, namely thermal or mechanical therapies, should be added to ensure sustained hemostasis. In terms of safety, no major complications, including intestinal obstruction or vascular embolization, have been observed in more than 150 patients treated with TC-325 for GIB.
Adherent clots
The approach to ulcers with an overlying clot is to irrigate the lesion in an attempt to dislodge the blood clot and reveal the underlying stigma. Vigorous washing for up to 5 minutes is effective in removing the clot in about 40% of lesions. Ulcers with clots resistant to such manipulation are defined as adherent clots (Forrest IIB). These lesions can be further managed by mechanical clot removal without disrupting the pedicle to expose the ulcer base (after preliminary dilute epinephrine injection around the ulcer), followed by endoscopic therapy according to the stigmata of recent bleeding.
The optimal management algorithm for adherent clots remains controversial, as RCTs comparing medical with endoscopic therapy have yielded diverging results. Indeed, some trials have shown that endoscopic therapy decreases rebleeding in adherent clots, whereas others, including one trial using contemporary high-dose intravenous PPI, have demonstrated low rebleeding rates with acid suppression alone. A previous meta-analysis combining 6 studies with 240 patients suggested a significant reduction in rebleeding with endoscopic hemostasis. However, this work was criticized because of statistical shortcomings and marked heterogeneity of the combined trials, namely differing PPI regimens used in the control arms of the included studies. A more recent meta-analysis failed to detect significant benefits of endoscopic therapy over no endoscopic therapy for adherent clots in terms of rebleeding, need for surgery, or mortality. Consensus guidelines state that endoscopic therapy can be considered while acknowledging that intensive PPI therapy alone may be adequate.
Proton-pump inhibitors postendoscopic therapy
PPI use in ulcer bleeding improves outcomes. A Cochrane analysis of 31 trials with 5792 patients revealed that PPI use decreases rebleeding (OR 0.45; 95% CI 0.36–0.57), the need for surgery (OR 0.56; 95% CI 0.45–0.70), or repeat endoscopy (OR 0.40; 95% CI 0.30–0.53) regardless of endoscopic hemostasis. A reduction in mortality was achieved only in trials conducted in Asian patients, and more importantly, in patients with active bleeding or nonbleeding visible vessel who had first undergone successful endoscopic hemostasis and who received high-dose intravenous PPIs (80 mg bolus and 8 mg/h infusion for 72 hours).
Another meta-analysis assessing high-dose intravenous PPI as an adjunct to endoscopic hemostasis further supports the observed decrease in rebleeding (OR 0.40; 95% CI 0.28–0.59), surgery (OR 0.43; 95% CI 0.24–0.76), and mortality (OR 0.41; 95% CI 0.20–0.84). Alternative dosing and oral routes have not yielded consistent results to allow for confident routine recommendations of such regimens. All patients with HRS should be treated with high-dose intravenous PPI after endoscopic hemostasis for 72 hours, a period during which most rebleeding occurs. This practice also appears to be cost-effective.
Second-look endoscopy
A second-look endoscopy refers to the performance of a preplanned repeat endoscopy 16 to 24 hours following the initial endoscopy, aimed at assessing the need for further endoscopic therapy directed toward any remaining HRS, if present. This practice has previously shown rebleeding benefits, although many of the trials did not include concomitant PPI use and used epinephrine monotherapy, a technique now shown to be suboptimal. In fact, in the setting of high-dose intravenous PPI, second-look endoscopy did not provide added benefits. A recent meta-analysis acknowledges the decreased rebleeding conferred by second-look endoscopy in certain high-risk patients with active bleeding, in the absence of high-dose PPI, but discourages against its routine practice in all patients. Consequently, consensus guidelines have concluded that contemporary data do not favor such practice. While further studies are needed to delineate high-risk groups that may benefit from routine second-look endoscopy, the added cost it would entail also needs to be considered. A rebleeding rate exceeding 31% may be needed to offset the cost of a second endoscopy.
In settings where the primary hemostasis is uncertain, and where the initial evaluation was incomplete because of blood clots, repeat endoscopy may be warranted.
Predictors of failure of endoscopic therapy
Rebleeding after endoscopy occurs in 10% to 15% patients presenting with NVUGIB, although reported values vary in the literature. Data specific to ulcer bleeding vary between 6.3% and 25.2%. Several independent predictors of endoscopic failure have been identified based on both pre-endoscopic and endoscopic features. These predictors include hemodynamic instability (OR 3.30; 95% CI 2.57–4.24), transfusion requirement, and hemoglobin less than 10 g/dL (OR 1.73; 95% CI 1.14–2.62). Active bleeding at endoscopy (OR 1.70; 95% CI 1.31–2.22), the location of bleeding ulcer in either the posterior duodenal wall (OR 3.83; 95% CI 1.38–10.66) or gastric high lesser curvature (OR 2.86; 95% CI 1.69–4.86), in addition to greater ulcer size, particularly larger than 2 cm, also contribute to higher rebleeding rates.
In patients with clinical evidence of rebleeding, a second endoscopy should be attempted to achieve hemostasis. Indeed, comparing endoscopic retreatment with surgery, rates of successful hemostasis in the endoscopy arm reached 73% (35 of 48 patients), with overall lower complication rates, but comparable mortality and length of stay. Failure of repeat endoscopy should be treated with transcatheter arterial embolization or surgery. Details pertaining to such therapies are discussed in articles elsewhere in this issue.
Helicobacter pylori testing
Helicobacter pylori eradication has been demonstrated in a meta-analysis to be significantly more effective than PPI therapy alone in preventing rebleeding from peptic ulcer disease. As such, consensus guidelines have recommended that all patients with peptic ulcer bleeding undergo H pylori evaluation, with subsequent eradication if tested positive. It is important that if the evaluation for H pylori is negative in the acute setting, repeat testing is indicated. Indeed, a systematic review of 23 studies showed that diagnostic tests for H pylori infection (including serology, histology, urea breath test, rapid urease test, stool antigen, and culture) are associated with high positive predictive values (0.85–0.99) but low negative predictive values (0.45–0.75) in the setting of acute GIB, with 25% to 55% of H pylori– infected patients yielding false-negative results. This high false-negative rate in acute bleeding may be related, at least in part, to an alkaline milieu imparted by the presence of blood in the gastric lumen, resulting in proximal migration of the bacterium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







