Endoscopic bariatric therapy consists of devices or procedures for primary weight loss or weight regain after Roux-en-Y gastric bypass that are placed or done endoscopically. In most cases, they result in less weight loss, but fewer complications than bariatric surgery; and more weight loss than lifestyle therapy or weight loss medications. These therapies are important advances to treat patients with obesity. This article focuses on therapies or devices with US Food and Drug Administration approval or those with current or planned US pivotal trials.
Key points
- •
Multiple devices and procedures for primary treatment of obesity and treatment of weight regain after Roux-en-Y gastric bypass (RYGB) are now available.
- •
In many cases, efficacy of these procedures and devices has been demonstrated with randomized sham-controlled or nonsham-controlled trials.
- •
Multiple gastric and small bowel devices for weight loss are currently being evaluated or have US pivotal trials planned for US Food and Drug Administration approval.
- •
Endoscopic revision of the gastrojejunostomy for weight regain after RYGB is an effective treatment for weight regain as shown in a randomized sham-controlled trial for patients with dilation of the gastrojejunostomy.
Introduction
Endoscopic bariatric therapy (EBT) has the potential to play a significant part in both the primary and secondary therapy of obesity. EBT may have more effectiveness than lifestyle therapy alone or medications, and while EBT generally is less effective than bariatric surgery, it has fewer complications and is less expensive. In 2015, the US Food and Drug Administration (FDA) approved 2 new endoscopic bariatric devices for primary obesity treatment: the ReShape Integrated Dual Balloon System (ReShape Dual Balloon, ReShape Medical, San Clemente, California) and the Orbera Intragastric Balloon System (Apollo Endosurgery, Austin, Texas). Several therapies are available for gastrojejunostomy revision for weight regain after Roux-en-Y gastric bypass (RYGB).
This article will discuss devices or techniques with FDA approval (including devices that have general FDA approval for nonspecific uses in the gastrointestinal [GI] tract), as well as those with currently running or planned US pivotal trials for FDA approval. EBT methods, their results and risks, are outlined.
Primary therapy
The American Society for Gastrointestinal Endoscopy (ASGE) recently released a position statement supporting the use of EBT in conjunction with a multidisciplinary weight loss program for long-term obesity treatment. EBT can be considered in those who have failed weight loss or maintenance with lifestyle intervention alone and meet BMI criteria for particular treatment modalities, or Have medical conditions that require weight loss for additional therapy (eg, bridge therapy to weight loss surgery). Current approved and investigational devices for primary EBT include space-occupying devices, tissue apposition devices, and nutrient-diverting devices.
Intragastric Balloons
The use of intragastric balloon (IGB) therapy for obesity was first described in 1982, and the Garren-Edwards gastric bubble (GEGB), an endoscopically-placed air-filled balloon, was approved for use by the FDA in 1985. However, multiple adverse events were reported with this device, including gastric mucosal injury, small bowel obstruction following spontaneous balloon deflation and migration, and poor efficacy compared with lifestyle modification alone in several trials. The GEGB was withdrawn from the US market in 1992. Several design flaws likely contributed to its ultimate failure. It was made from polyurethane, which deflated too easily, and had a cylindrical shape with edges, which led to mucosal injury. Additionally, it filled only to a volume of 220 mL, whereas a volume of 400 mL has been shown to be the minimum necessary to reduce food intake. Subsequent efforts to design IGBs have drawn from the experience with the GEGB to create safer and more effective devices. This article focuses on the IGBs that are available in the United States or for which pivotal trials are either ongoing or planned in the United States. Devices are summarized in Table 1 .
| Device Name | Structure/Materials | Fill Type and Volume | Method for Placing and Removing | Dwell Time |
|---|---|---|---|---|
| Orbera (Apollo Endosurgery) | Single silicone balloon | 400–700 mL saline | Endoscopic | 6 mo |
| ReShape Duo (ReShape Medical) | Two tethered silicone balloons | 750 or 900 mL saline | Endoscopic | 6 mo |
| Spatz (Spatz Medical) | Single silicone balloon, attached catheter | 400–1000 mL saline, adjustable | Endoscopic | 12 mo |
| Obalon (Obalon Therapeutics) | Lightweight balloon enclosed in capsule | 250 mL gas, can swallow up to 3 balloons | Swallowed, removed endoscopically | 6 mo |
| Elipse (Allurion Technologies) | Lightweight balloon enclosed in capsule | 550 mL saline | Swallowed, degrades, passes in stool | 4 mo |
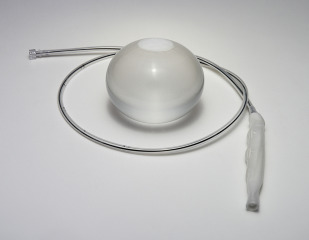
Fluid-filled single balloon: Orbera
The most-studied and widely used IGB is the Orbera IGB, which was introduced in 1991 and originally known as the BioEnterics Intragastric Balloon (BIB, Allergan, Irvine, California) ( Fig. 1 ). The Orbera IGB is a single spherical balloon made of silicone that is placed endoscopically and filled with saline. The deflated elastic balloon with attached catheter is passed into the stomach alongside a standard upper endoscope, and position in the body is confirmed by direct visualization. The device is then inflated with 400 to 700 mL of saline, often with 2 to 10 mL of methylene blue solution added. The methylene blue serves to alert the patient if the balloon has deflated; it is absorbed systemically and excreted in the urine, turning the urine green–blue in color. The device remains in place for 6 months and is removed endoscopically. The Orbera balloon was approved by the FDA for treatment of obesity in 2015.
Recent meta-analyses have been conducted to evaluate efficacy and safety of the device. The ASGE Bariatric Endoscopy Task Force systematically reviewed 82 studies in 2015. Seventeen of these studies reported percent excess weight loss (%EWL) at 12 months after device implantation, or 6 months after removal, with a total of 1638 patients studied. These patients averaged a 25.4% EWL, with a 95% confidence interval (CI) of 21.5% to 29.4%. Only 3 studies had adequate control groups, with Orbera patients (n = 131) losing an average of 26.9% more weight than control patients (n = 95), with 95% CI of 15.6% to 38.2%. A large randomized controlled trial has been conducted in the United States, but the results have not yet been published.
The Orbera balloon has also been well studied with respect to safety. In the ASGE task force meta-analysis, 68 studies reported rates of adverse events. Pain and nausea were most common, occurring in 33.7% of patients, with a 7% rate of early removal. More serious complications were rare, with ulceration in 2% of patients, migration in 1.4% of patients, small bowel obstruction of 0.3% of patients, and gastric perforation in 0.1% of patients. Four total deaths were reported, related to perforation or aspiration. Most studies did not report timing of adverse events, but in 1 larger retrospective series, 51 balloon leaks or deflations were observed, with 49 of these occurring after the recommended 6-month period.
Fluid-filled dual balloon: ReShape duo
The ReShape Integrated Dual Balloon System is an endoscopically placed device consisting of 2 equal-sized silicone balloons connected by a flexible shaft ( Fig. 2 ). After placement of the deflated dual-balloon device, each balloon is filled separately with either 375 or 450 mL of saline with methylene blue, for a total volume of 750 or 900 mL depending on the height of the patient. This design is intended to prevent migration of the device, since unintentional deflation of 1 balloon would leave the other still intact and holding the device in place. The device is removed endoscopically at 6 months. ReShape was approved by the FDA for treatment of obesity in 2015.
The ReShape IGB was evaluated in the REDUCE (A Prospective, Randomized Multicenter Study to Evaluate the Safety and Efficacy of the ReShape Duo Intragastric Balloon System in Obese Subjects) randomized sham-controlled pivotal trial. The completed study included 326 participants with BMI 30 to 40 kg/m 2 at 8 US study centers, and all subjects received monthly lifestyle therapy coaching. At 24 weeks, Duo subjects had an average %EWL of 25.1% compared with 11.3% in Diet patients ( P = .004) on intention-to-treat analysis. An average of 66% of weight lost at time of balloon removal was maintained 24 weeks later.
Like other IGBs, abdominal pain, nausea, and vomiting were common, particularly in the first week after implantation. Early retrieval for intolerance occurred in 9% of patients within the first 2 months, which prompted a reduction in fill volume to 375 mL per balloon for patients shorter than 5 feet 4 inches tall, with subsequent reduction in early retrievals. In addition, gastric ulceration was seen in 39% of treatment patients, resulting in a minor design change to smooth the device tip, which reduced the ulcer rate to only 10%; most ulcers were noted incidentally on 6 month balloon removal. Deflation occurred in 6% of patients, with no migrations or obstructions observed.
A subsequent clinical case series in Spain demonstrated 15.4% total body weight loss (TBWL) at 6 months in 60 patients. Only 1 early removal for intolerance occurred, and 1 leak occurred. Fourteen patients had ulcerations on balloon removal, and 1 patient had a gastric perforation. Of note, the old balloon design with higher rate of ulceration was used in all of these patients.
Fluid-filled adjustable balloon: Spatz
The Spatz Adjustable Balloon System (Spatz Medical, Great Neck, New York) is an endoscopically placed, saline-filled single intragastric balloon ( Fig. 3 ). Unlike other available IGBs, which are inflated to desired volume on implantation, the Spatz has an attached inflation tube that remains in the stomach with the balloon and can be extracted out through the mouth to adjust the balloon volume during therapy to improve tolerance or efficacy. The Spatz balloon is not approved by the FDA but is approved in Europe for 12 months of therapy.
At present, there are limited safety and efficacy data on the Spatz IGB. A study of 73 patients in the United Kingdom showed a 45.7% EWL. Four patients had the balloon removed due to intolerance; 10 patients had the volume adjusted downward with comparable weight loss outcomes, and 45 patients had the volume adjusted upward for decline in weight loss with mostly good efficacy. Complications included catheter migration and impaction resulting in need for surgical extraction in 3 cases, which led to a change in the device design with a softer catheter. No difference in weight loss was seen in an 80-patient case–control study between 12 months of Spatz IGB and 12 months of Orbera treatment using 2 sequential balloons. Safety concerns have been raised, with higher overall rates of complications compared with Orbera and reports of delayed gastric or bowel perforation, but the frequency of such complications is not yet known.
Gas-filled swallowed balloon: Obalon
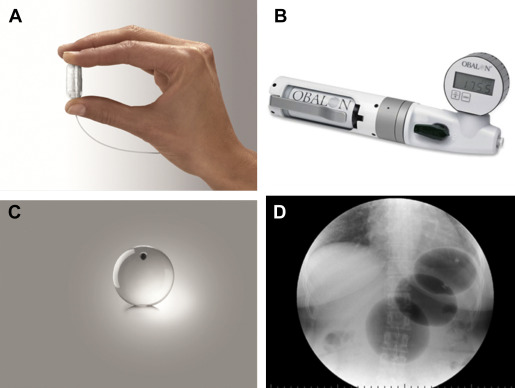
The Obalon Gastric Balloon (OGB, Obalon Therapeutics, Carlsbad, California) differs from the previously discussed IGBs in that it is enclosed in a gelatin capsule, which is swallowed by the patient under fluoroscopic visualization ( Fig. 4 ). The capsule has an attached catheter that extends through the esophagus and out of the mouth, and is used to inflate the balloon with 250 mL of a nitrogen-mix gas. A pilot study involving 17 patients was undertaken in Europe, with up to 3 balloons given per patient, swallowed individually at 4-week intervals, and removed endoscopically at 12 weeks. Average excess weight loss at 12 weeks was 36%, with no serious adverse events reported. Most patients experienced transient abdominal pain and nausea after balloon administration. A large randomized sham-controlled trial in the United States with balloons in place for 6 months has recently been completed, but results have not yet been published.
Fluid-filled swallowed balloon: Elipse
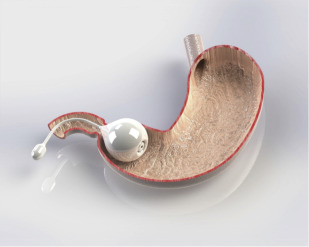
The Elipse IGB (Allurion Technologies, Wellesley, Massachusetts) is enclosed within a capsule with an attached catheter and swallowed under radiographic guidance ( Fig. 5 ). After placement is confirmed, the balloon is filled with 550 mL of fluid and remains in the stomach for 4 months. The balloon is made with a material that degrades over the 4-month period on the internal side of the release valve. Once the material degrades, the release valve opens, causing a catastrophic deflation of the balloon, which then passes on its own. An 8-patient pilot study using a prototype demonstrated no adverse events other than nausea, vomiting, and cramping, and patients lost an average of 12.4% of excess weight over 6 weeks. Results of a small multicenter study were reported at a recent conference, but have not yet been published.
TransPyloric Shuttle
The TransPyloric Shuttle (BAROnova, Goleta, California) consists of a spherical bulb attached by a flexible cord to a smaller cylindrical bulb ( Fig. 6 ). The device is placed endoscopically and assembled in the stomach. The large bulb rests in the antrum, with the smaller bulb crossing into the duodenum. This creates intermittent obstruction at the pylorus leading to delayed gastric emptying and longer periods of satiety. One study of 20 participants showed promising results, with mean EWL of 25% at 3 months and 41% at 6 months and gastric ulcers in 50% of patients with early device removal in 2 patients due to symptomatic gastric ulcers. The ENDObesity II multicenter study is currently underway, with no results yet published.
Tissue Apposition
Endoscopic sleeve gastroplasty
In endoscopic sleeve gastroplasty (ESG), gastric volume is reduced by using endoscopically placed sutures to create a gastric sleeve similar to a sleeve gastrectomy. This is accomplished using the Overstitch endoscopic suturing device (Apollo Endosurgery), an FDA-approved commercial device for the purposes of tissue apposition compatible with a double-channel therapeutic gastroscope ( Fig. 7 ). Full-thickness stitches can be placed in interrupted or running patterns and sutures reloaded without removal of the endoscope. The sleeve is created by apposing the gastric wall along the greater curvature.









