Background
The concept of screening is to identify a disease at a stage in its natural history where treatment can be applied in order to prevent death or suffering. For prostate cancer, this is challenging because the variable natural history of the disease differs markedly from slow-growing indolent tumors and highly aggressive and potentially fatal forms. Benefits of screening should be an increased but not excessive rate of earlystage detected cancers, a decrease in metastatic cancer and a reduction in cancer-specific mortality. Several authors have asked whether screening for prostate cancer is a suitable strategy to reduce prostate cancer mortality. Recently, two randomized prostate cancer screening trials published their final data. The European Randomized Study of Screening for Prostate Cancer (ERSPC) showed that prostate-specific antigen (PSA)-based screening lowers prostate cancer mortality while the study conducted in the United States, the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial showed no benefit in prostate cancer mortality [1,2]. The ERSPC was conducted in eight European countries and enrolled 267,994 men 55–74 years of age, while the PLCO trial enrolled 155,000 women and men 55–74 years of age. The main purpose of these two trials was to evaluate whether population-based screening reduces the mortality from prostate cancer, with an acceptable level of quality-of-life aspects and associated costs. Possible disadvantages of screening are overdetection with resultant overtreatment, increased costs, side effects and complications. This chapter discusses the current evidence available on early detection and screening for prostate cancer.
Clinical question 26.1
What is the sensitivity and specificity of PSA as an early detection tool for prostate cancer?
Literature search
We searched Medline using the term “PSA” and other relevant keywords (“prostate cancer,” “screening,” “early detection,” “sensitivity,” “specificity,” “randomized controlled trials,” “clinical studies,” “case–control cohorts”). We limited the searches to English-language articles published between January 1980 and March 2009; non-English-language studies were excluded, because their quality is difficult to evaluate.
The evidence
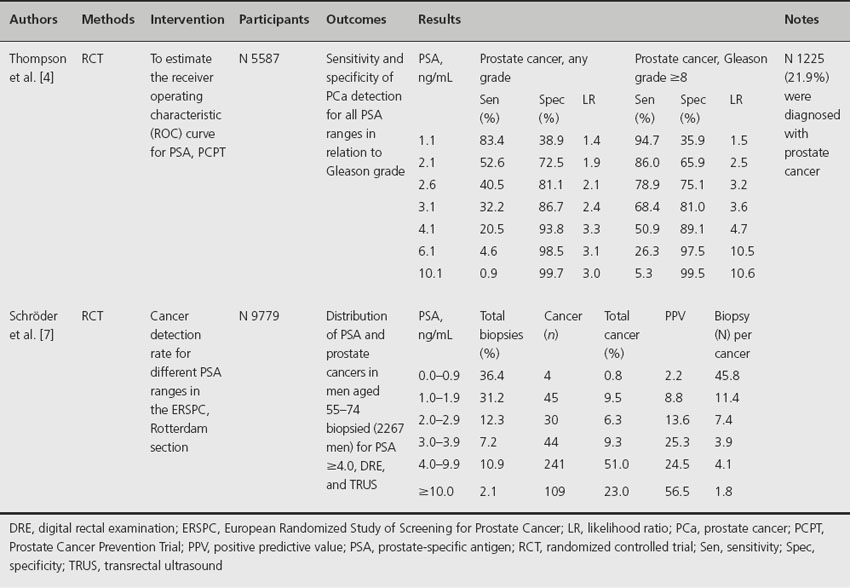
The main tool in screening for prostate cancer, prostate-specific antigen, was first described in 1979. It is a human protein secreted by prostate epithelial cells and is perhaps more a specific organ marker than a tumor marker since prostatitis, benign prostate hyperplasia (BPH) and other conditions can also increase PSA. PSA has a large intraindividual day-to-day variation and is influenced by bodyweight [3]. Partly due to the fact that PSA is not a cancer-specific measurement, no clear threshold level exists for PSA sensitivity and specificity [4]. In clinical practice, recommendations based on PSA vary between 2.0 and 4.0 ng/mL. Krumholtz et al. recommended a cut-off point of 2.6 ng/mL as an indicator for biopsy after they established a prostate cancer detection rate of 22% in the 2.6–4.0 ng/mL PSA range [5]. Thompson et al. demonstrated, after biopsying men in low PSA ranges, that there is no cut-point of PSA with equal sensitivity and specificity for monitoring healthy men for prostate cancer [4,6]. In this trial, after biopsying 5587 men, a prostate cancer prevalence of 15% in men with PSA 4.0 ng/mL and lower was found; 15% of those men with prostate cancer had high-grade cancer (Gleason score ≥ 7) [6]. According to these study results, a physician who requires 80% confidence in not missing a prostate cancer should apply a PSA cut-off value of 1.1 ng/mL as an indication for biopsy, which would result in 60% unnecessary biopsies.
The lack of sensitivity and specificity of PSA in low ranges is confirmed in the ERSPC tria l[7]. In Table 26.1, the continuum of prostate cancer risk for different PSA ranges is presented for the ERSPC and the Prostate Cancer Prevention Trial (PCPT) [4,7]. As shown, sensitivity decreases with increasing PSA level, while specificity increases. Besides this, Thompson et al. proved that the sensitivity and specificity of PSA are improving for high-grade cancer compared with any prostate cancer, demonstrating that PSA is a better marker of high- than of low-grade disease [4]. Considering the continuum of prostate cancer risk over different PSA ranges, one should question whether it is necessary to detect all prostate cancers present in all PSA ranges. After comparing the biopsy rates of men in the PCPT placebo arm with the cancer detection rates, interval cancers and prostate cancer deaths of three subsequent screening rounds, applying a PSA cut-off of 3.0 ng/mL as biopsy indication in the ERSPC trial, Schröder et al. concluded that if we biopsied all men regardless of PSA level, a very unfavorable balance between men biopsied and the detection of one (deadly) prostate cancer would occur [8]. This is in line with earlier publication of the ERSPC study in which was concluded that among men whose cancer was not detected at initial screening by applying a 4.0 ng/mL PSA cut-off, it was unlikely that a substantial number would progress to noncurable stages at the time of repeat screening 4 years later [9,10]. Overall, the authors concluded that, although the sensitivity and specificity of PSA are not ideal, a serum PSA cut-off of 3.0 ng/mL is not too high combined with a 4-year screening interval. Besides this, men presenting with PSA values between 2.0 and 2.9 ng/mL at initial screening might be primarily targeted [8].
Table 26.1 The continuum of prostate cancer risk for different PSA ranges, presented for the ERSPC and the PCPT
In addition to Catalona et al. recommending a biopsy in men with PSA higher than 2.5 ng/mL, they suggest that prostate cancer screening should begin at age 40 years to establish a baseline PSA measurement and to assess risk for prostate cancer. Because PSA elevation at age 40–50 is more strongly associated with a later diagnosis of prostate cancer than an elevation of PSA at age 60, a primary goal of PSA testing in men ≤ 50 should be to stratify cancer risk at an early age rather than to detect prostate cancer [11–13]. In future, a risk-adjusted screening strategy based on a single baseline PSA measured at age 40–50 may be possible and prevents men receiving many unnecessary biopsies.
Clinical question 26.2
What is the additional value of digital rectal examination (DRE) and transrectal ultrasound (TRUS) in low PSA ranges for early detection and screening of prostate cancer?
Literature search
We searched Medline using the terms “digital rectal examination” and “transrectal ultrasound” with other relevant keywords (“prostate cancer,” “screening,” “early detection,” “PSA,” “sensitivity,” “specificity,” “randomized controlled trials,” “clinical studies,” “case–control cohorts”). We limited the searches to English-language articles published between January 1980 and March 2009; non-English-language studies were excluded, because the quality of these studies is difficult to evaluate.
The evidence
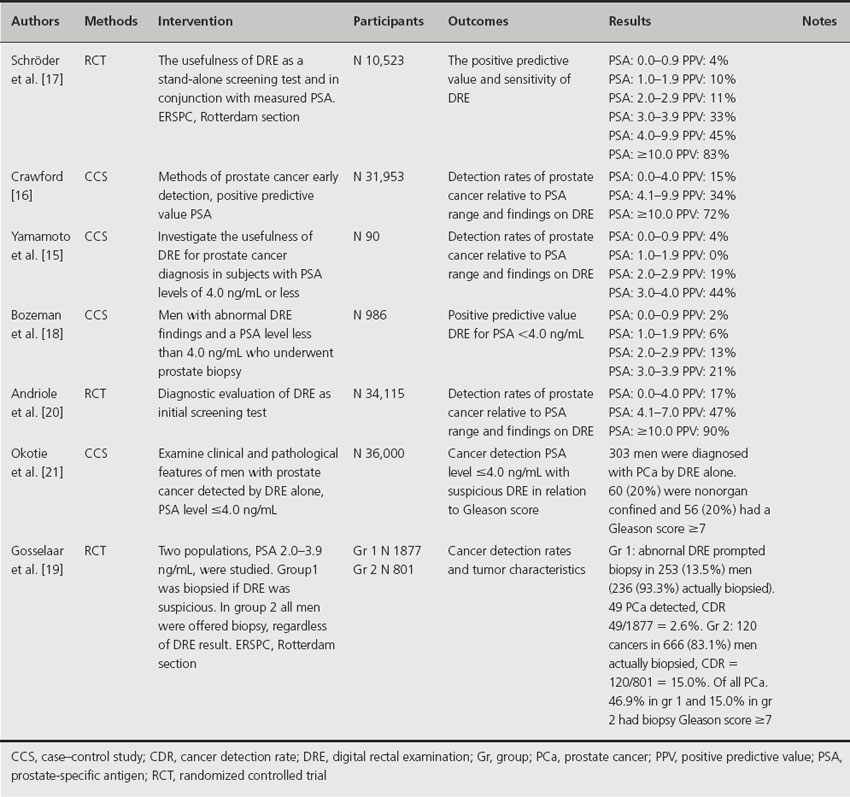
Although DRE is still widely used for the diagnosis of prostate cancer, its central role is superseded by the widespread application of serum PSA. DRE is possible due to the anatomical position of the prostate in the pelvis, with easy access for palpation using a finger placed per rectum. In screening and early detection programs for prostate cancer, the value of DRE remains controversial because it is not standardized and varies widely among physicians [14]. Table 26.2 provides an overview of the positive predictive value for DRE in different PSA ranges [15–21]. DRE has a low sensitivity and predictive value in men with low PSA levels where it should be most useful. At serum PSA levels below 3.0 ng/mL, 289 rectal examinations are required to find one case of clinically significant disease, and 96 rectal examinations are needed to diagnose prostate cancer of any size, grade or stage [22]. According to these results, and the fact that tumors detected by DRE had the lowest pathological stage, the ERSPC has omitted DRE as an initial screening test [23]. In addition, Gosselaar et al. reported that an abnormal DRE was not a significant predictor for diagnosing prostate cancer on repeat screenings [24]. Although an abnormal DRE should no longer be an independent indicator for biopsy in low PSA ranges, Okotie et al. found that a substantial proportion of cancers detected by DRE alone at PSA levels < 4.0 ng/mL have clinically aggressive features; nearly 20% had a Gleason score ≥ 7 [21]. Furthermore, Gosselaar et al. point out that potentially aggressive cancers (Gleason ≥ 7) are more prevalent among men who have an abnormal DRE compared to normal DRE at PSA levels ≥ 3.0 ng/mL. Okotie et al. suggested that omission of DRE from screening protocols might compromise treatment outcomes because many of the cancers detected by DRE alone are potentially curable but may have worse outcomes by the time PSA also reaches a higher level. However, Okotie et al. conducted a univariate analysis for suspected DRE only and not a multivariate analysis with consideration of PSA. Consequently, the additional value of DRE over PSA would be small since Thompson et al. showed that 15% men with PSA ≤ 4.0 ng/mL had cancer on biopsy [6].
Table 26.2 Positive predictive value for prostate cancer detection, DRE for different PSA ranges
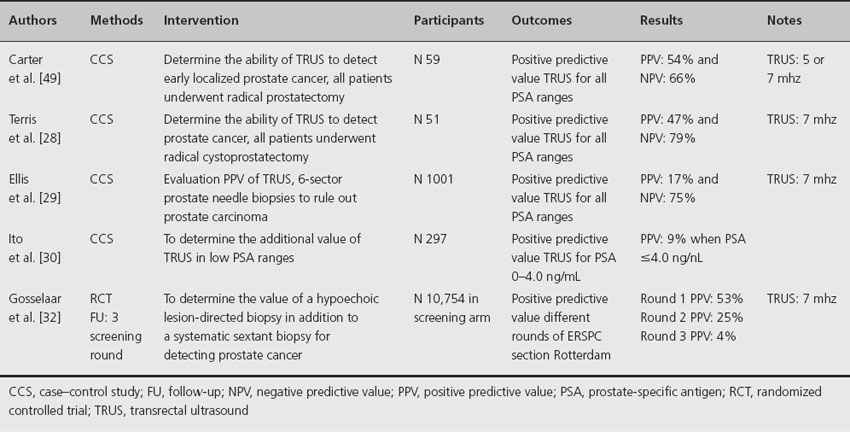
Transrectal ultrasound is in widespread use by urologists although less often as a primary prostate cancer screening tool. In the ERSPC, TRUS is used to guide biopsies of the prostate gland in patients with elevated serum PSA, with an additional biopsy of possible hypoechoic lesions [23]. TRUS is not useful to identify prostate cancer because of the frequent multifocality of cancer within the prostate, the variable sonographic appearance of prostate tumors, the poor specificity of focal ultrasonic abnormalities, and the substantial percentage of isoechoic prostate cancers (which cannot be differentiated from adjacent benign tissues with imaging) [25]. Some studies have shown that hypoechoic lesion-directed biopsy is as sensitive as a biopsy from an isoechoic region [26]. Table 26.3 shows the positive and negative predictive value of TRUS-guided biopsy of hypoechoic lesions in different studies [27–32]. The only studies in which sensitivity and specificity are not relative (all patients included who had a radical prostatectomy to ensure the presence of cancer) were those by Carter et al. [27]. and Terris et al. [28] who reported sensitivities of 52% and 53.3% and specificities of 68% and 75% respectively for all PSA ranges. One study determined the additional value of TRUS in low PSA ranges; they presented a low positive predictive value (9% for PSA ≤ 4.0 ng/mL) [30]. Gosselaar et al. reported the value of additional biopsies of suspicious lesions in a screening situation with systematic sextant biopsy [32]. They concluded that it would be preferable in men previously not biopsied; the additional biopsy core was statistically significantly more often positive for cancer than the random sextant biopsy cores. However, in future this value will decline because of the more common extended biopsy schemes and the decline in the percentage of men with no previous biopsy.
Table 26.3 Positive predictive value of TRUS for prostate cancer detection
Comment
Although the risk of (high-grade) prostate cancer increases with the level of serum PSA, there is no serum PSA level below which there is no risk of having prostate cancer. Currently we can conclude that serum PSA is the most accurate of the three diagnostic tests. The overall findings suggest that the DRE is a poor screening tool for prostate cancer, and increasing levels of serum PSA have been demonstrated to be more important in detecting prostate cancer. Therefore DRE should not necessarily be recommended as a primary screening tool since it caused an unfavorable number of biopsies to detect one cancer in low PSA ranges. However, its role in combination with PSA for diagnosis is necessary, as it gives essential clinical information for staging and treatment decision making.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








