Chapter 8 Drug-induced and toxic liver disease
1 Drug-induced liver injury (DILI) accounts for 7% of reported drug adverse effects, 2% of hospitalizations for jaundice, 1% of cases of acute liver failure (ALF), and most cases of hepatitis in patients older than 50 years of age. Ten percent of those with jaundice will die.
2 The spectrum of DILI ranges from subclinical liver disease with mildly elevated liver chemistry test results to subacute liver failure and ALF requiring liver transplantation. DILI can mimic almost every type of acute and chronic liver disease.
3 Many different medications and toxins have been implicated in causing ALF, and the prognosis is poor without liver transplantation.
4 Over-the-counter preparations and herbal medications may have significant hepatotoxicity, and their use should be determined in cases of unexplained acute or chronic liver disease.
5 Early suspicion of DILI is essential because morbidity is greatly increased if the medication is continued after symptoms develop or liver chemistry test abnormalities appear.
Overview
1. Drug-induced liver injury (DILI) is one of the most common causes of elevated liver chemistry values.
3. The Drug-Induced Liver Injury Network (DILIN) was created by the National Institutes of Health in 2003. Initial DILIN findings showed that 73% of cases resulted from taking a single prescription medication, 9% were attributable to herbal or dietary supplements, and 18% resulted from taking multiple agents.
4. Among patients with DILI caused by a single prescription drug, the major offending agents were antimicrobials (46%), central nervous system agents (15%), immunomodulating agents (5%), analgesics (5%), and lipid-lowering agents (3%).
6. The incidence of DILI is greater in older individuals, probably related to altered pharmacokinetic factors.
7. Polypharmacy may contribute to an increased risk of adverse drug reactions through alterations of cytochrome P-450.
8. Although most DILI cases in children are mild, pediatric patients have the potential to progress to ALF.
9. Children with viral infection have an unusual sensitivity to aspirin (Reye’s syndrome); however, the most common agents causing DILI in children are antiepileptic drugs, such as valproate, and psychotropic agents.
Clinical Presentation
1. DILI may occur as an unexpected idiosyncratic reaction to a medication’s therapeutic dose or as an expected consequence of the agent’s intrinsic toxicity.
2. Hepatotoxicity may be the only manifestation of the adverse drug effect, or it may be accompanied by injury to other organ systems or by systemic manifestations.
3. Acute liver injury may develop within days of ingestion of a known hepatotoxin or after several weeks of taking a drug that provokes an immunoallergic reaction. Liver tests may have a necroinflammatory, cholestatic, or a mixed pattern with features of both parenchymal and cholestatic injury.
 Aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) and lactate dehydrogenase may be elevated 10 to 100 times the upper limit of normal (ULN) in acute hepatocellular injury, whereas alkaline phosphatase levels are usually less than 3 times the ULN.
Aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) and lactate dehydrogenase may be elevated 10 to 100 times the upper limit of normal (ULN) in acute hepatocellular injury, whereas alkaline phosphatase levels are usually less than 3 times the ULN.
 Cholestatic drug injury resembles obstructive jaundice in its clinical manifestations and biochemical parameters. Serum alkaline phosphatase, gamma glutamyltranspeptidase, and direct bilirubin are variably elevated with or without aminotransferase elevation (usually no higher than five to eight times ULN).
Cholestatic drug injury resembles obstructive jaundice in its clinical manifestations and biochemical parameters. Serum alkaline phosphatase, gamma glutamyltranspeptidase, and direct bilirubin are variably elevated with or without aminotransferase elevation (usually no higher than five to eight times ULN).
 Subclinical hepatic injury reflected only by minor liver enzyme elevation (e.g., AST and ALT in the range of 100 to 250 U/L) is a common phenomenon and may not worsen and may even subside despite continued administration of a medication.
Subclinical hepatic injury reflected only by minor liver enzyme elevation (e.g., AST and ALT in the range of 100 to 250 U/L) is a common phenomenon and may not worsen and may even subside despite continued administration of a medication.
 Aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) and lactate dehydrogenase may be elevated 10 to 100 times the upper limit of normal (ULN) in acute hepatocellular injury, whereas alkaline phosphatase levels are usually less than 3 times the ULN.
Aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) and lactate dehydrogenase may be elevated 10 to 100 times the upper limit of normal (ULN) in acute hepatocellular injury, whereas alkaline phosphatase levels are usually less than 3 times the ULN. Cholestatic drug injury resembles obstructive jaundice in its clinical manifestations and biochemical parameters. Serum alkaline phosphatase, gamma glutamyltranspeptidase, and direct bilirubin are variably elevated with or without aminotransferase elevation (usually no higher than five to eight times ULN).
Cholestatic drug injury resembles obstructive jaundice in its clinical manifestations and biochemical parameters. Serum alkaline phosphatase, gamma glutamyltranspeptidase, and direct bilirubin are variably elevated with or without aminotransferase elevation (usually no higher than five to eight times ULN). Subclinical hepatic injury reflected only by minor liver enzyme elevation (e.g., AST and ALT in the range of 100 to 250 U/L) is a common phenomenon and may not worsen and may even subside despite continued administration of a medication.
Subclinical hepatic injury reflected only by minor liver enzyme elevation (e.g., AST and ALT in the range of 100 to 250 U/L) is a common phenomenon and may not worsen and may even subside despite continued administration of a medication.TABLE 8.1 Clinicopathologic patterns of drug-induced liver injury∗
| Disorder | Hepatotoxic agents |
|---|---|
| Acute | |
| Hepatitis-like syndromes (acute necroinflammation) | Dapsone, disulfiram, isoniazid, carbamazepine NSAIDs, allopurinol, bupropion, lisinopril, losartan, paroxetine, phenytoin, sulfonamides, statins, trazodone, pyrazinamide, HAART, valproic acid |
| Fulminant hepatic failure | Acetaminophen, fialuridine (FIAU), halothane, isoniazid, sustained-release niacin, nitrofurantoin, propylthiouracil, valproic acid, flutamide |
| Cholestasis | Ampicillin, chlorpromazine, prochlorperazine, cimetidine, ranitidine, estrogens, cytarabine, trimethoprim–sulfamethoxazole, thiabendazole, tolbutamide, anabolic steroids, erythromycins |
| Mixed necroinflammatory and cholestatic | Carbimazole, chlorpropamide, dicloxacillin, methimazole, azathioprine, naproxen, phenylbutazone, sulindac, phenytoin, thioridazine, captopril, cyproheptadine, enalapril, fosinopril, irbesartan, terbinafine, phenobarbital |
| Granulomatous hepatitis | Allopurinol, dapsone, diazepam, diltiazem, hydralazine, penicillin, phenylbutazone, phenytoin, quinidine, procainamide, clopidogrel, sulfonamides |
| Macrovesicular steatosis | Alcohol, corticosteroids, L-asparaginase, methotrexate, nifedipine, tamoxifen |
| Microvesicular steatosis | Alcohol, amiodarone, aspirin, zidovudine, didanosine, piroxicam, tetracyclines, tolmetin, valproic acid |
| Budd–Chiari syndrome | Estrogens |
| Ischemic necrosis | Cocaine, sustained-release niacin, methylenedioxyamphetamine |
| Chronic | |
| Chronic active hepatitis | Alpha-methyldopa, nitrofurantoin, oxyphenisatin |
| Fibrosis/cirrhosis | Alcohol, alpha-methyldopa, isoniazid, methotrexate |
| Peliosis hepatis | Anabolic/androgenic steroids, azathioprine, hydroxyurea, oral contraceptives, tamoxifen |
| Phospholipidosis | Amiodarone, perhexiline, diltiazem, nifedipine |
| Primary biliary cirrhosis | Chlorpromazine, haloperidol, prochlorperazine |
| Sclerosing cholangitis | Floxuridine (FUDR) by hepatic artery infusion |
| Steatohepatitis | Amiodarone, diethylstilbestrol, tamoxifen, irinotecan |
| Sinusoidal obstruction syndrome | Azathioprine, busulfan, cyclophosphamide, daunorubicin, oxaliplatin, pyrrolizidine alkaloids, 6-thioguanine |
| Autoimmune hepatitis | Minocycline, statins |
| Hepatoportal sclerosis | Didanosine |
| Nodular regenerative hyperplasia | Didanosine, azathioprine, 6-thioguanine, 6-mercaptopurine |
| Vanishing bile duct syndrome | Azithromycin, amoxicillin-clavulanic acid, anabolic steroids, allopurinol |
| Oncogenic | |
| Cholangiocarcinoma | Thorotrast |
| Focal nodular hyperplasia | Estrogens, oral contraceptives |
| Hepatic adenoma | Estrogens, oral contraceptives |
| Hepatocellular carcinoma | Alcohol, anabolic/androgenic steroids |
| Hepatoblastoma | Estrogens |
| Angiosarcoma | Arsenic, vinyl chloride, Thorotrast |
| Inflammatory pseudotumor | Anabolic steroids |
HAART, highly active antiretroviral therapy; NSAIDs, nonsteroidal anti-inflammatory drugs.
Characterization of Drug-Induced Liver Injury
Idiosyncratic Hepatotoxicity
1. This occurs unpredictably in a small number of recipients of a medication and accounts for most cases of DILI. Some dose dependency exists, and toxicity is not reliably reproduced in laboratory animals. Examples of agents include isoniazid (INH), sulfonamides, valproate, and phenytoin.
3. Susceptibility results from an interplay among factors such as the toxic potential of the drug, environmental and host genetic risk factors that determine drug disposition, and metabolism and tissue susceptibility to toxicity.
4. Immunologically mediated injury can be accompanied by a mononucleosis-like illness and extrahepatic hallmarks of generalized hypersensitivity such as fever, rash, and eosinophilia; these features usually develop after a sensitization period of several weeks.
Pathophysiology
1. The liver is exposed to high concentrations of ingested drugs, particularly those with a high first-pass metabolism.
2. Hepatic uptake of drugs may occur by specific transport mechanisms; most drugs are lipophilic and diffuse across the hepatocellular sinusoids.
3. Many of the mechanisms in the pathophysiology of DILI at the molecular level are shown in Table 8.2.
4. Normally, the liver metabolizes drugs to more polar forms, thus facilitating their excretion in aqueous fluids. Sometimes these metabolites may be toxic (e.g., in acetaminophen overdose), but they are generally converted to less toxic compounds by detoxification enzymes.
5. Individual susceptibility to drug hepatotoxicity is influenced by multiple variables that affect the biotransformation of drugs, and usually more than one of these is involved in any one patient (Table 8.3).
TABLE 8.2 Mechanisms of drug-induced liver injury occurring at the molecular level
| Peroxidation of lipids |
| Denaturation of protein |
| Adenosine triphosphate depletion |
| Mitochondrial dysfunction |
| Free radical generation |
| Electrophilic radical generation and hapten formation |
| Biotransformation through cytochrome P-450 |
| Binding of active metabolites to nuclear or cytoplasmic molecules |
| Binding or blockage of transfer RNA |
| Binding or blockage of bile transporters |
| Attachment to membrane receptors |
| Disruption of calcium homeostasis |
| Disruption of the hepatocellular cytoskeleton |
TABLE 8.3 Factors influencing an individual’s susceptibility to drug-induced liver injury
| Age |
| Long-term alcohol use |
| Drug–drug interactions |
| Duration of use and total dose of drug |
| Enzyme induction |
| Enzyme polymorphism |
| Ethnic and racial factors |
| Gender |
| Human leukocyte antigen (HLA) type |
| Nutritional status |
| Pregnancy |
| Renal function |
| Systemic disease |
| Underlying liver disease |
Biotransformation
This is a process by which therapeutic agents are rendered more hydrophilic, thus facilitating their excretion from the body. Biotransformation takes place in several steps, classified as phase 1, phase 2, and phase 3 reactions.
Phase 1 Reactions
1. These are mediated by cytochrome P-450, are primarily oxidative, and yield active intermediate metabolites that may be responsible for liver injury. The cytochrome P-450 family of isoenzymes found primarily within the endoplasmic reticulum results in aliphatic and aromatic hydroxylation, dealkylation, or dehydrogenation. Products of these reactions may sometimes undergo further metabolism through phase 2 reactions.
Phase 2 Reactions
1. These are mainly conjugative, converting the active metabolite to nontoxic, more hydrophilic products by linkage with glutathione, sulfate, or glucuronide. This is the only step required for the hepatic metabolism of some compounds; however, most drugs first undergo cytochrome P-450 metabolism.
Diagnosis of Drug-induced Liver Injury
1. A detailed drug history, including dosage, duration of therapy, and other concomitantly administered drugs, is essential.
2. Other causes of liver disease must be excluded by careful assessment of clinical, radiologic, histologic, biochemical, and serologic findings.
4. Elevation of serum lactate dehydrogenase levels is more indicative of toxic liver injury than of viral-related disease, although this finding is nonspecific.
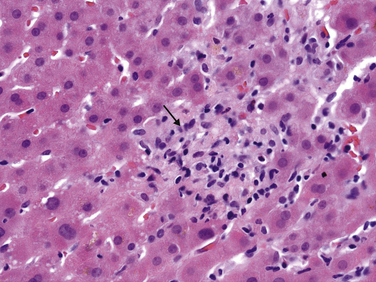
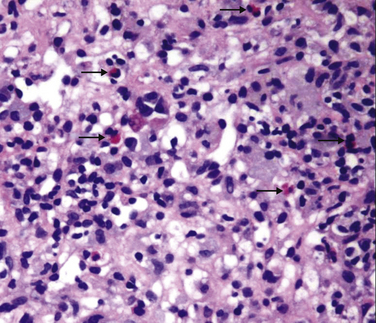
5. Nonspecific histologic lesions suggestive of drug injury include granulomas (Fig. 8.1), eosinophils within an inflammatory infiltrate (Fig. 8.2), a sharp zone of demarcation between necrosis and unaffected parenchyma, and a disproportionately severe degree of damage in relation to the patient’s condition and the extent of liver chemistry test abnormalities.
Hepatotoxicity of Specific Medications
Acetaminophen (Paracetamol, Tylenol)
1. This is typically well tolerated without side effects. Overdose is the most common cause of DILI leading to ALF.
2. The amount ingested as a single dose required for hepatic injury is quite variable. A toxic dose may be 10 to 20 g, whereas in alcoholic patients it can be as low as 5 to 10 g.
3. Acetaminophen is present in many over-the-counter (e.g., Nyquil) and prescription (e.g., Vicodin) preparations.
4. The greatest risks for hepatotoxicity are influenced by the dose of acetaminophen ingested and the interval between drug ingestion and administration of the antidote.
5. Alcoholism is a significant risk factor for toxicity, and appreciable liver injury sometimes occurs with therapeutic use or unwitting overdose (“therapeutic misadventure”). Malnutrition or fasting may play a role in acetaminophen hepatotoxicity by reducing glutathione stores.
6. The use of concurrent medications that induce cytochrome P-450 may heighten the risk and severity of liver injury associated with acetaminophen overdose.
Clinical Phases after Massive Ingestion
2. Cessation of gastrointestinal symptoms is followed by a period of well-being for approximately 48 hours. Right-sided abdominal pain, oliguria, elevated liver chemistry test results, and prolonged prothrombin time then occur.
3. Hepatic necrosis occurs 3 to 5 days after ingestion. Aminotransferase levels may peak at greater than 20,000 U/L. Renal failure from proximal and distal renal tubular damage occurs in up to 20% of patients, and ALF develops in up to 30%.
Prognosis
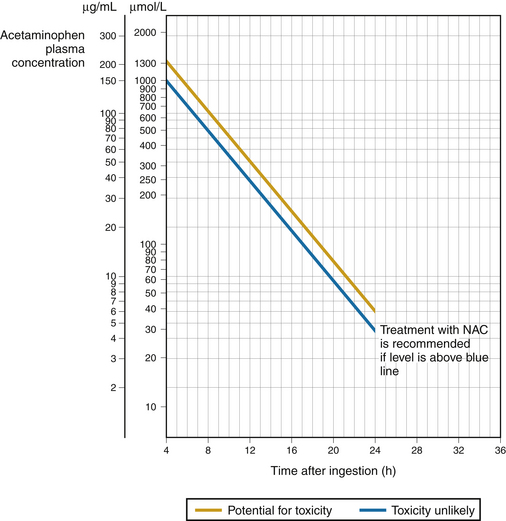
1. Risk of liver injury can be assessed based on the serum acetaminophen level obtained more than 4 hours after ingestion (Fig. 8.3).
2. Criteria for predicting death or the need for liver transplantation:
 Prothrombin time international normalized ratio (INR) greater than 6.5 and serum creatinine higher than 3.4 mg/dL in patients with stage 3 or 4 encephalopathy
Prothrombin time international normalized ratio (INR) greater than 6.5 and serum creatinine higher than 3.4 mg/dL in patients with stage 3 or 4 encephalopathy
 Prothrombin time international normalized ratio (INR) greater than 6.5 and serum creatinine higher than 3.4 mg/dL in patients with stage 3 or 4 encephalopathy
Prothrombin time international normalized ratio (INR) greater than 6.5 and serum creatinine higher than 3.4 mg/dL in patients with stage 3 or 4 encephalopathy