Distal Subtotal Gastrectomy and D1 Resection
K. Roggin
Mitchell C. Posner
Gastric adenocarcinoma is the fourth most common malignancy and the second leading cause of death worldwide annually. In the United States, it remains a relatively rare cancer (in 2010, approximately 22,000 new cases were diagnosed). Gastric carcinoma is associated with chronic exposures to environmental carcinogens (Helicobacter pylori, nitrosamines, tobacco exposure), inflammatory conditions of the stomach (atrophic gastritis), pernicious anemia, blood type A, and less commonly, genetic mutations in the E-cadherin gene, CDH-1 (hereditary diffuse gastric cancer). The relatively poor overall survival reported in most Western series is likely influenced by the high percentage of patients who are diagnosed with locally advanced or metastatic disease. Symptoms remain nonspecific early in the course of the disease, but advanced tumors can cause GI hemorrhage, gastric outlet obstruction, and profound weight loss. Curative treatment requires an appropriate gastrectomy with adequate regional lymphadenectomy. Peri- or postoperative treatment with chemotherapy and/or radiation has been shown to reduce recurrence and improve survival. Tumor location, clinical stage of disease, and patient performance status influence the decision to perform a subtotal versus total gastrectomy. Lymphatic metastases should be treated with an appropriate lymphadenectomy for optimal locoregional control of disease. Early-stage cancers may be cured by complete endoscopic mucosal resection (e.g., T1a cancers) or surgical gastrectomy; chemotherapy and/or radiation are effective adjuvant treatments that have been associated with improved disease-free and overall survival in stage II and III disease. Optimal management should be individualized with the input of a multidisciplinary tumor board. Two treatment paradigms are accepted as standards of care:
Perioperative chemotherapy using EOX (Epirubicin–Oxaliplatin–Capecitabine) or equivalent chemotherapy for three cycles (nine weeks) before and after radical gastrectomy (MAGIC trial regimen).
Subtotal or total gastrectomy followed by chemotherapy and external beam radiation (McDonald regimen, SWOG 0116).
NCCN guidelines (www.nccn.org) suggest a comprehensive staging workup for patients with resectable gastric cancer.
Comprehensive history and physical examination
Complete upper endoscopy defining the location of the tumor within the stomach, extent of intragastric spread, and relationship of the cancer to the gastroesophageal junction.
All biopsies should be reviewed by a dedicated GI pathologist to determine the histologic subtype (intestinal, Lauren’s diffuse type, signet ring cell adenocarcinoma, adenosquamous carcinoma) and degree of differentiation.
Endoscopic ultrasonography should be considered in select patients who are candidates for neoadjuvant chemotherapy protocols. This modality is accurate at assessing the depth (T stage) of invasion, presence of metastatic regional lymphadenopathy, and distant metastatic disease to the liver or peritoneal cavity (liver metastases, peritoneal implants, and/or ascites).
Contrast-enhanced, triphasic (pre-, arterial-weighted, and portovenous phases) multirow detector computed tomography of the chest, abdomen, and pelvis.
Positron-emission tomography (PET scans) remains an experimental diagnostic staging modality in gastric adenocarcinomas, as only two-thirds of these mucin-producing or signet ring adenocarcinomas have the ability to concentrate the radiotracer fluoro-deoxyglucose. PET–CT fusion scanning has been reported to improve the diagnostic accuracy compared with either CT or PET scans alone.
Staging laparoscopy ± peritoneal washings (cytology) should be considered in patients with ≥T3 or node-positive cancers; as many as 20 to 30% of patients with negative radiographic and endoscopic imaging will have occult M1 or stage IV disease on laparoscopy.
Peritoneal cytology appears to be an independent predictor associated with death from gastric adenocarcinoma.
Complete surgical resection of gastric cancers is the only treatment modality associated with long-term survival. The tumor stage, location, and performance status of the patient influence the optimal type of resection (subtotal vs. total gastrectomy). Two randomized prospective trials have shown that the estimated overall survival after distal subtotal gastrectomy is equivalent to total gastrectomy for distal gastric cancers. Total gastrectomy is associated with higher postoperative complication rates, more frequent concomitant splenectomy, and longer inpatient length of stay. In addition, it is often associated with significant long-term protein-calorie malnutrition and functional impairment. Proximal gastrectomy has not been rigorously compared with total gastrectomy, but it offers a reasonable alternative to total gastrectomy for cancers of the cardia and gastroesophageal junction (GEJ). In general, this procedure has a higher frequency of recalcitrant postoperative biliary reflux. Radical gastrectomy requires a comprehensive understanding of the arterial supply of the stomach and duodenum (Fig. 15.1), lymphatic drainage basins, and physiologic consequences of decreasing the volume of the gastric reservoir. The optimal extent of regional lymphadenectomy remains controversial. Two landmark-randomized controlled trials failed to show a short-term survival benefit with D2 lymphadenectomy. Results from both a recent trial by Wu et al. and a 15-year re-analysis of the Dutch gastric cancer trial suggest a small absolute survival benefit for patients who were treated with extended lymphadenectomy (>D1).
Operative principles include the following:
Complete laparoscopic and open assessment of occult, sub-radiographic metastases to the liver, peritoneal cavity, adrenal glands, and distant lymphatic basins.
Complete resection of the primary tumor with at least 5-cm proximal and distal margins.
Appropriate regional lymphadenectomy as indicated by the location of the primary tumor (proximal, middle, and distal stomach) and stage of disease. In the absence of clinical lymphadenopathy in the D2 drainage basins (celiac axis distribution), a complete D1 lymphadenectomy may be sufficient treatment. Resecting at least 15 lymph nodes appears to ensure adequate staging accuracy.
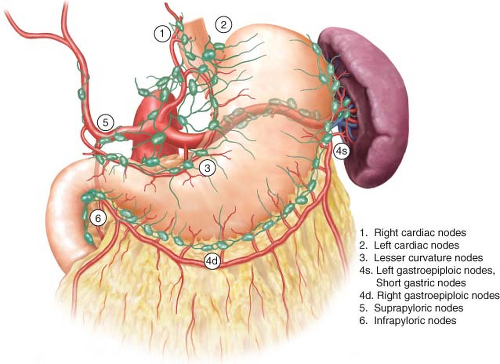
D1 lymphadenectomy involves removal of the perigastric lymph nodes along the lesser curvature (stations 1, 3, and 5) and greater curvature (stations 2, 4, and 6) (Fig. 15.2).
D2 lymphadenectomy extends the lymphatic sampling to all of the lymph nodes around the celiac axis and its named vessels (stations 7–11).
Gross and histologic intraoperative margin assessment.
Reconstruction of GI tract continuity with appropriate conduit to maximize function and reduce the incidence of postgastrectomy syndromes.
Operative Positioning and Setup
Patients are positioned on the operating room table in the supine position with appropriate padding. Sequential compression devices and a single dose of 5,000 units of subcutaneous heparin are administered prior to induction of anesthesia to reduce the incidence of perioperative thromboembolic events. An oro- or nasogastric tube and Foley catheter are placed into their respective locations after the patient has been sedated and successfully intubated. Complete pharmacologic neuromuscular blockade is essential to maximize operative exposure and minimize incision length. Broad-spectrum antibiotics covering gastric flora (e.g., second-generation cephalosporins) are given intravenously
within 1 hour prior to the incision; antibiotics are routinely re-dosed within one-half life (generally 4 to 6 hours) if needed. The patient’s left arm is usually padded and tucked to facilitate the placement of the retractor arm. The skin is prepared with a chlorhexidine solution and allowed several minutes to completely dry before draping the patient. An antibiotic-impregnated impermeable barrier is placed on the abdominal skin.
within 1 hour prior to the incision; antibiotics are routinely re-dosed within one-half life (generally 4 to 6 hours) if needed. The patient’s left arm is usually padded and tucked to facilitate the placement of the retractor arm. The skin is prepared with a chlorhexidine solution and allowed several minutes to completely dry before draping the patient. An antibiotic-impregnated impermeable barrier is placed on the abdominal skin.
Operative Technique
Diagnostic Laparoscopy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree