1. Oxford classification: split system
M
Mesangial hypercellularity
≤0.5 (M0) or ≥0.5 (M1)
E
Endocapillary hypercellularity
Absent (E0) or present (E1)
S
Segmental sclerosis
Absent (S0) or present (S1)
T
Tubular atrophy/interstitial fibrosis
<25 % (T0), 26-50 % (T1), or >50 % (T2)
2. Japanese histological grading classification: lumped system
Histological grade
No of lesions*/total no. of glomeruli
Active lesions only
Active lesion + chronic lesion
Chronic lesion only
H-Grade I
0–24.9 %
A
A/C
C
H-Grade II
25–49.9 %
A
A/C
C
H-Grade III
50–74.9 %
A
A/C
C
H-Grade IV
75 %
A
A/C
C
Table 5.2
Pathological parameters of Oxford and JHGC
Oxford | JHGC | ||
|---|---|---|---|
Active glomerular lesions | Mesangial hypercellularity | ○ | × |
Endocapillary hypercellularity | ○ | × | |
Cellular or fibrocellular crescent | × | ○ (late progressor) | |
Chronic glomerular lesions | Global sclerosis | × | ○ (early and late progressor) |
Segmental sclerosis | × | ○ (early progressor) | |
Segmental sclerosis or adhesion | ○ | × | |
Fibrous crescent | × | ○ (early progressor) | |
Adhesion | × | × | |
Tubulointerstitium | Tubular atrophy/interstitial fibrosis | ○ | × |
Vascular lesions | Interlobular artery | × | × |
Afferent artery | × | × |
5.2.1 Oxford Classification
5.2.1.1 Development
An international committee of pathologists and nephrologists from four continents were first convened in 2004 for the purpose of developing a new, evidence-based, international consensus histologic classification for IgAN. The 265 cases were assembled including biopsies from 206 adults and 59 children and were from 17 different centers based in eight countries and four continents.
The following rigorous procedure was taken to develop the evidence-based classification (Fig. 5.1). First, the histological parameters which are necessary to take in the lesions of IgAN were discussed and determined. Using these selected lesions, a score sheet was produced to quantify these selected histological parameters consisting of glomerular, tubulointerstitial, and vascular lesions. Second, on the basis of data-using score sheets, reproducibility was tested by the Intraclass Correlation Coefficient (ICC), and the parameters with poor reproducibility were excluded for further analysis. Third, the histological parameters with reasonable reproducibility were assessed by linear regression analysis and Cox regression analysis to determine if they were independent predictors for the renal functional outcome for a minimum of 3 years and/or until an end point of end-stage renal disease (ESRD) or ≥50 % decline in estimated glomerular filtration rate (eGFR).


Fig. 5.1
Comparison of the producing procedure between Oxford and JHGC
As a consequence, three histologic parameters were selected as independent predictors within this classification (M: mesangial hypercellularity, S: segmental sclerosis, and T: tubular atrophy/interstitial fibrosis (TA/IF)). One additional histologic parameter—endocapillary hypercellularity (E) in >= 1 glomerulus—was not significantly correlated with the above outcomes by multivariate analysis but was strongly correlated with response to immunosuppressive therapy and hence this additional parameter was included in the classification. Finally, in order to dichotomize each of the selected parameters to produce a split classification system, cutoff points were determined by ROC (Receiver Operating Characteristic) analysis. Thus, the recommendation of the classification with IgAN includes four independently reported histologic parameters (M, E, S, and T): M0 or M1, indicating mesangial hypercellularity in ≤50 % versus >50 % of glomeruli; E0 or E1, indicating endocapillary hypercellularity in 0 versus ≥1 glomeruli; S0 or S1, indicating segmental sclerosis in 0 versus ≥1 glomeruli; and T0, T1, or T2, indicating TA/IF in ≤25 %, 26–50 %, or >50 % of renal cortex, respectively. They produced a four-parameter dichotomous scoring system to describe IgAN lesions.
5.2.1.2 Validation Studies of Oxford Classification
The Oxford Histologic Classification has gained a significant level of worldwide acceptance and has been the subject of several single-center or multicenter validation studies. Validating the classification in a diverse range of cohorts treated in different ways is important to confirm that the classification can be widely applied. Among validation studies, the selected independent predictors for renal functional outcome by multivariate analysis were M, S, and T [8] in one, M and S in one, 2 [9], S and T in two [10–12], M and T in two [13, 14], E and T in one, [15], only T in three, [16–18], only CT in one [11], and none in one study [19], respectively (Fig. 5.2).
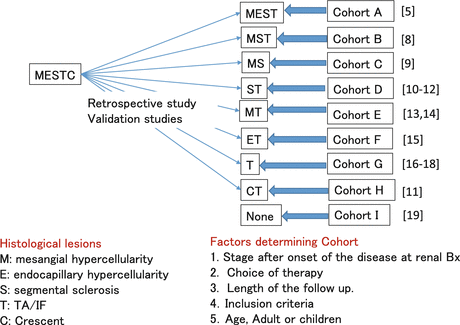

Fig. 5.2
Evidence-based Oxford classification (Split system)
Confirmatory Studies
Coppo et al. compared the applicability of the Oxford classification to predict renal outcomes in 59 children as compared with the 206 adults included in the original Oxford study cohort. M1 vs. M0 and S1 vs. S0 were associated with an increased rate of renal functional decline. In contrast, E1 vs. E0 was not. These findings are similar to those reported in the entire Oxford cohort and suggested that selected parameters were not influenced by the patient age. However, the effects of the T variable were not directly examined because there were too few children with tubulointerstitial lesions to draw conclusions [9]. European validation study of the Oxford classification of IgA (VALIGA) also confirmed the results from the original Oxford classification study using 1,147 patients from 13 European countries. Over a median follow-up of 4.7 years, M, S, and T lesions independently predicted the loss of eGFR and a lower renal survival. In individuals with eGFR less than 30 ml/min per 1.73 m2, the M and T lesions independently predicted a poor survival [8].
Using a cohort of 187 adults and children with IgAN from 4 North American centers, S and T scores were confirmed as independent predictors of renal functional decline, and this study also confirmed that endocapillary proliferation (E1) was associated with an increased rate of renal functional decline in patients not treated with immunosuppression but not in patients receiving immunosuppression, similar to findings in the original Oxford cohort. M score was not an independent predictor of renal functional decline in the study [10]. Katafuchi et al. studied 702 Japanese adults and children by multivariate analysis and found that S and T lesions were associated with ESRD. M1 showed a trend toward high risk of ESRD compared with M0. E score showed no significant association with the development of ESRD. When crescent (C) was added to the multivariate analysis, C and T, but not S, were significantly associated with the development of ESRD. They documented also that an association of crescent with ESRD was not observed in patients who met the inclusion criteria of Oxford cohort but was evident in those who did not [11].
Shi et al. evaluated 410 Chinese patients and found that S and T scores were independent predictors of ESRD. Furthermore, patients having >25 % of glomeruli with endocapillary proliferation (termed E25), crescents, and M1 were more likely to be treated with immunosuppressive agents, but none of these parameters were independent predictors of prognosis [12]. Zeng et al. found that only M1 and T scores were associated with an increased rate of eGFR decline or reduced renal survival from a combined event by multivariate analysis using 1,026 adult IgAN patients from 18 centers in China [13]. Shima et al. defined as a decline in eGFR to <60 ml/min per 1.73 m2 in 161 consecutive Japanese IgAN children (<20 years old) and found that M and T scores (but not S) and crescents in >30 % of glomeruli (but not in ≥1 glomerulus) were significant predictors of renal outcome in multivariate analyses [14]. Lee et al. reported on 69 adult patients followed for >36 months and found that E1 and T1/T2 lesions independently predicted a ≥50 % decline in eGFR or ESRD, even though patients with E1 lesions received more immunosuppression than those with E0 [15].
Divergent Studies (One or Less Parameters)
Four studies found only one of the Oxford M, S, and T scores, most often the T score, to be independently predictive of clinical outcome. El Karoui et al. studied 128 adult patients and found only T score predicted rate of eGFR decline, and only M score was associated with doubling of serum creatinine or ESRD by multivariate analysis [16]. In the study by Kang et al. using 197 Korean adult patients, they showed that only T lesions predicted a 50 % reduction in eGFR or ESRD. Lee et al. reported on 69 adult patients followed for >36 months. E1 and T1/T2 lesions independently predicted a ≥50 % decline in eGFR or ESRD, even though patients with E1 lesions received more immunosuppression than those with E0 [17]. Le et al., in a study of 218 Chinese children, found that only T1/2 was an independent predictor of poor outcome in multivariate analysis [18]. Finally, Alamartine et al. studied 183 French adults including very mild and very severe forms of the disease for an average of 77 months; only baseline eGFR was predictive of reaching this end point by multivariate analysis [19].
As shown above, the Oxford classification offers a simple approach for predicting renal outcome by describing the presence or absence of active (M, E) and chronic lesions (S, T). It is also the only current glomerular disease classification that was developed in a truly evidenced-based manner.
5.2.2 Japanese Histological Grade Classification (JHGC)
5.2.2.1 Development
As mentioned in the introduction, within Japan, an alternate classification system has been developed. This came from the work of the IgAN Study Group of the Progressive Renal Diseases Study Committee under the auspices of the Ministry of Health, Labor and Welfare. A multicenter case-control study on IgAN was conducted to develop an evidence-based clinicopathological classification for predicting long-term renal outcome.
This working group consisted of 16 centers. The cohort was drawn from patients seen in Japanese hospitals. Lesions were assessed independently by two pathologists, and if they did not agree, lesions were reviewed by both pathologists until a consensus was reached. The initial panel of histological parameters examined was very similar to that of the Oxford classification. During a median follow-up of 9.3 years after biopsy, 49 out of 287 patients (19 %) progressed to ESRD. The associations between pathological variables and the need for chronic dialysis were examined by multivariate logistic regression analysis separately in patients who required dialysis earlier than 5 years (early progressors) and those who required dialysis within 5–10 years (late progressors) after biopsy. Independent pathological variables predicting progression to ESRD were global sclerosis, segmental sclerosis, and fibrous crescents for early progressors and global sclerosis and cellular/fibrocellular crescents for late progressors (Fig. 5.3). Four histological grades (HGs), such as HG 1, HG 2, HG 3, and HG 4, were established corresponding to <25 %, 25–49 %, 50–74 %, and ≥75 % of glomeruli, exhibiting cellular or fibrocellular crescents, global sclerosis, segmental sclerosis, or fibrous crescents. Eleven (7 %) patients in HG 1, 12 (16 %) in HG 2, 13 (31 %) in HG 3, and 13 (68 %) in HG 4 progressed to ESRD. Multivariate logistic analysis revealed that the risk of progression to ESRD was significantly higher in HG 2, 3, and 4 than in HG 1 (odds ratio, 2.4, 5.7, and 27.6 vs. 1.0). Age, mean arterial pressure, and urinary protein excretion were higher, while eGFR was lower in higher grades, suggesting that our histological grading agrees with the prognostic clinical features of IgAN at the time of diagnosis. The risk of progression to ESRD and the rate of GFR decline were significantly greater in higher grades, indicating that the grading can identify the magnitude of the risk of progression to ESRD as well as the deterioration rate of renal function [7]. In addition to the grading system, this classification allowed for active (A) chronic lesions (C) and mixed (A/C) to be produced as a subgroup of each grade which allows identification of a balance between activity and chronicity.
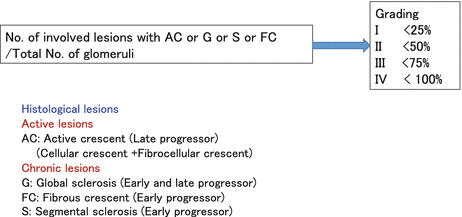

Fig. 5.3
Japanese Histological Grading Classification (Lumped system)
5.2.2.2 Validation Studies
Compared to the Oxford classification scheme, a limited number of validation studies have been conducted, and these have been confined to Japanese cohorts. Sato et al. validated the JHGC in a Japanese single-center cohort [20]. This was a retrospective study in 198 Japanese adult patients with IgAN. Clinical parameters including blood pressure, urinary protein, eGFR, and outcomes were evaluated in these patients. The glomerular lesion percentage score (GLPS) [number of glomeruli with cellular crescents, fibrocellular crescents, global sclerosis, segmental sclerosis, or fibrous crescents per number of total obtained glomeruli] was assessed in each patient and categorized into histologic grades of HG1 (<25 %), HG2 (25–49 %), and HG3/4 (≥50 %). Associations of GLPS (or HG) with disease progression (50 % eGFR decline or ESRD-requiring dialysis) within 10 years after biopsy and the rate of annual eGFR decline were examined. During a median follow-up period of 12.0 years after biopsy, disease progression occurred in 12.8 % (12/94) of HG1 patients, 32.3 % (21/65) of HG2 patients, and 46.2 % (18/39) of HG3/4 patients. The risk of disease progression was significantly higher in the HG2 and HG3/4 groups than in the HG1 group (odds ratios: 3.3 and 5.9 vs.1). A higher GLPS was significantly associated with a higher risk of disease progression and a greater annual eGFR decline. JHGC based on GLPS (or HG) was well correlated with long-term prognosis in the cohort of Japanese adult patients with IgAN [20].
5.3 Comparison Between Oxford Classification and Japanese Histological Grade Classification
Despite similar objective (the communication of complex pathological information using a simplified scoring system), the Oxford and Japanese histological grade classification schemes differ on a number of points, both in the methodological approach to developing the classification schemes and in the predictive pathological parameters suggested by each classification scheme. As these two classification systems coexist in Japan, there has been a great deal of motivation to compare the two in order to try and determine which approach may produce the most informative information for the sake of clinicians and ultimately for the benefit of the patients. In order to provide background to our subsequent discussion of the two classification schemes, the differences in their development and methodology are detailed below.
5.3.1 Differences in Development of Classification Schemes
5.3.1.1 Inclusion Criteria
The cohorts used in the studies were different between JHGC study and the Oxford classification study. The differences were influenced mainly by the inclusion criteria. The Oxford classification excluded patients with proteinuria less than 0.5 g/day and those with an initial eGFR of <30 ml/min per 1.73 m2, whereas in the JHGC study, there was no limitation for the cohort. This is a relevant variation as a significant fraction of Japanese patients had asymptomatic proteinuria with microhematuria or isolated microhematuria in their initial clinical presentation and were detected owing to the health-screening programs of Japan, whereas these patients would have been excluded from analysis within the Oxford cohort. This means that despite the overall Japanese cohort presenting with less severe IgAN lesions, it also included those with severe lesions, providing a wider range of IgAN lesions from which to develop its classification.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







