, Liqing Yao1 and Xinyu Qin2
(1)
Endoscopy Center, Zhongshan Hospital Fudan University, Shanghai, People’s Republic of China
(2)
General surgery, Zhongshan Hospital Fudan University, Shanghai, People’s Republic of China
Abstract
An endoscopic ultrasonography (EUS) uses an endoscope with an ultrasound probe attached to create real time detailed pictures of internal organs and structures
1.1 Endoscopic Ultrasonography
1.1.1 Overview
An endoscopic ultrasonography (EUS) uses an endoscope with an ultrasound probe attached to create real time detailed pictures of internal organs and structures.
In 1980, Dunagnoey and Strohm pioneered the use of EUS and after over 30 years of development, EUS has been playing an extremely important role in the diagnosis and treatment of digestive system diseases.
Commonly used ultrasonic endoscopes include ultrasonic gastroscopes, ultrasonic duodenoscopes and ultrasonic colonoscopes as well as mini ultrasonic probes that is passed either through an endoscopic biopsy channel for the diagnosis of subtle digestive tract wall and submucosal lesions or inserted into biliary and pancreatic ducts through duodenal papilla to perform detailed examination. Therapeutic indication include EUS-guided fine needle aspiration. Recent combination of color Doppler imaging with EUS enhanced accuracy of imaging and enabled the scanning of arterial and venous blood flows. Advancement in technology has allowed and its gradual application in clinical practice.
1.1.2 Classification of Ultrasonic Endoscopes
1.1.2.1 Dedicated Ultrasonic Endoscopes
Such type of ultrasonic endoscopes refer to those special endoscopes equipped with a mini ultrasonic probe permanently attached to their tip that allows detailed examination of superficial and deeper layers of digestive tract as well as adjacent structures.
1.1.2.2 Transendosopic Miniature Ultrasonic Probes
Such probe is 2 mm in diameter and can be inserted through the biopsy channel of endoscope into the lumen or even passed into biliary of pancreatic duct through duodenal papilla (IDUS). Its frequency ranges from 12 to 30 MHz.
1.1.2.3 Color Doppler Ultrasonic Endoscopes
Color Doppler enhances the capability of endosonography by visualizing the blood vessels in the digestive tract. It reduces risk of puncturing blood vessels during EUS-guided fine needle aspiration. It additionally help to assess vascular parameters of a lesion.
The integration of endoscopic color Doppler ultrasonography (ECDUS) with puncture-intended ultrasonic endoscopes are of the linear array scanning type, while some probes are of the central groove needle type. The advantage of such type of endoscopes is that they can clearly visualize the needle route as well as the blood vessels within the scanned area. In addition to diagnostic use, these are intended for therapeutic procedures such as aspiration biopsy and treatment of the biliary and cystic space-occupying lesions.
1.1.2.4 Puncture-Intended Ultrasonic Endoscopes
Such type of endoscopes is primarily intended for performing the EUS guided fine needle aspiration (EUS guided FNA) as well as paracentetic aspiration, injection, catheter drainage in digestive tract, liver or pancreas lesions.
1.1.2.5 3D-IDUS
Three dimensional IDUS (3D-IDUS) can enable the 3D imaging in gastrointestinal tract and biliary and pancreaticducts to further improve the resolution and diagnostic accuracy. 3D-IDUS is primarily intended for morphological visualization of the biliary duct and diagnosis of adjacent small tumors. The minimum section interval is 0.25 mm and the maximum sampling length is 40 mm.
1.1.3 Clinical Applications
1.1.3.1 TN Staging of Gastrointestinal Tumors
Generally, most of the tumors of the gastrointestinal tract can be easily diagnosed by conventional endoscopy, however, it is not possible to determine the depth of tumor infiltration or involvement of regional lymph node. The EUS allows TN staging of the digestive tract cancers hence provide guidance for the selection of appropriate treatment.
EUS help decide suitability of lesion for ESD treatment. ESD is only indicated for flat lesions and early cancers of the mucosa or SM1 layer (SM1) without regional lymph node metastasis. EUS can effectively determine the depth of lesion as well as involvement of regional lymph node.
Esophageal Carcinoma
EUS is reliably accurate (>80 %) in preoperative local staging of esophageal cancer and is considered superior to CT, MRI scan, in assessing depth of primary tumor infiltration. EUS is also reliable in staging a tumor and detecting mediastinal lymph or node metastasis (Fig. 1.1). Currently, EUS is the most accurate non-operative technique to determine the depth of tumor invasion. EUS can accurately predict whether esophageal cancer can be completely resected hence guiding appropriate treatment.
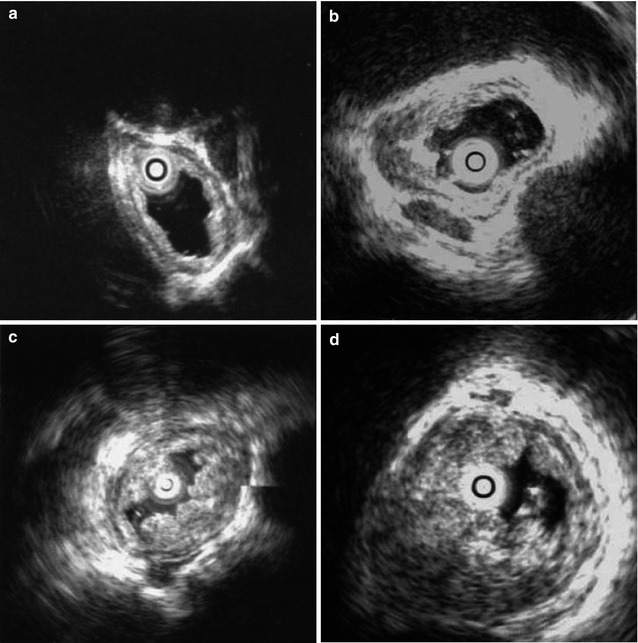


Fig. 1.1
TN staging of esophageal cancer by EUS. (a) Layer 1 is involved, Layer 2 is deficient and Layer 3 is partially invaded; (b) Layer 4 is slightly thickened and Layer 5 is not invaded; (c) The thickness of the esophageal wall is increased, but Layer 5 is intact; (d) Layer 5 is invaded; (e) Regional lymph nodes (+)
In case of an early esophageal cancer, EUS can differentiate whether the lesions are mucosal or infiltrate into the submucosa or muscularis propria. This provide the basis for minimally invasive endoscopic resection for mucosal lesion if no regional lymph nodes are involved.
Gastric Carcinoma
Intramucosal cancers without lymph node metastasis are increasingly resected endoscopically. Long-term follow-up data indicates that endoscopic therapy can achieve the same effects as surgical resection but with fewer complications. Therefore, preoperative staging prior to an endoscopic treatment is extremely important. EUS is extremely helpful in differentiating mucosal tumors from tumors invading the submucosa. EUS finding of involvement of Layer 3 of the gastric wall, is indicative of submucosal invasion hence surgical resection is indicated. EUS is relatively good in assessing I and IIc lesions, but is far from perfect to stage IIa type of lesions.
EUS can reliably stage a gastric cancer in terms of the depth of tumor invasion to the gastric wall, affected adjacent lymph nodes and direct infiltration to the adjacent organs (Fig. 1.2).
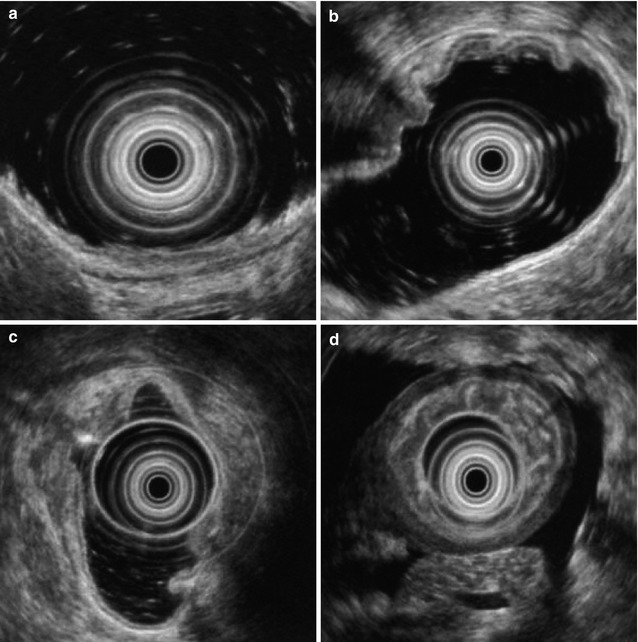


Fig. 1.2
TN staging of gastric cancer by EUS. (a) Early gastric cancer, hypoechoic and not penetrate the submucosa; (b) BorrmannII gastric cancer (fungating); (c) BorrmannIII gastric cancer (ulcerated); (d) BorrmannIV(infiltrating) gastric cancer, the gastric wall is significantly thickened, with structure destructed and ascitic fluid seen around the gastric wall; (e) Tumors have metastasized to the lymph nodes adjacent to the gastric wall
Colorectal Carcinoma
Flat lesions and early colorectal cancers are now treated with minimally invasive endoscopic therapy. EUS is being increasingly used in staging of colorectal cancer (Fig. 1.3). In case of low rectal cancer, preoperative EUS can determine the depth of tumor infiltration and whether adjacent organs have been invaded, thus providing information whether tumor can be resected and anus can be preserved. In case of early colorectal cancer, EUS can help determine lesions depth (infiltrated into the submucosa) to provide basis for suitability to select the minimally invasive endoscopic therapy.
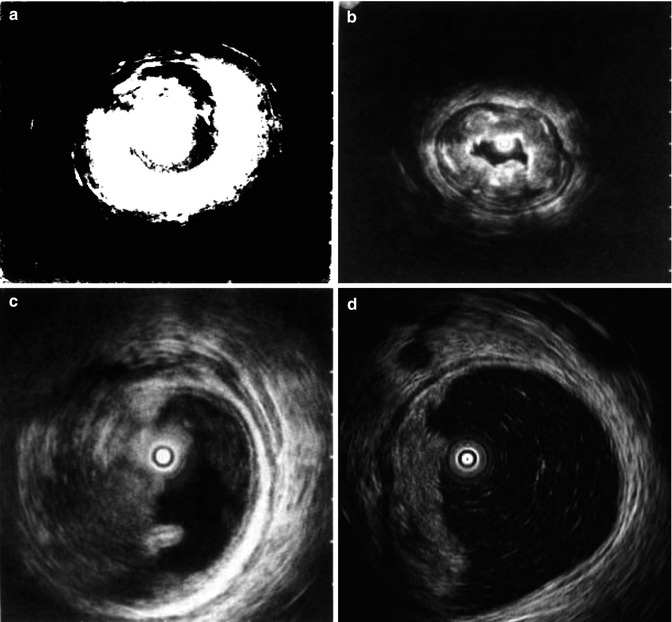

Fig. 1.3
TN staging of colorectal cancer by miniature EUS probe. (a) Tumors have affected Layer m and sm; (b) Tumors have affected Layer mp; (c) Tumors have affected the whole colonic layer; (d) Tumors have metastasized to the lymph nodes adjacent to the intestinal wall
1.1.3.2 Diagnosis of Gastrointestinal Submucosal Tumors
EUS can accurately assess submucosa tumors (SMTs) location, size and the layer of origin, and can also help characterize these lesions (Fig.1.4). Hypoechoic lesions derived from the muscularis mucosa (MM) or muscularis propria (MP) are either gastrointestinal leiomyoma or stromal tumors. Cysts, heterotopic pancreas and lipoma are derived from the submucosa. Carcinoid tumors, eosinophilic granuloma, myoblastoma, scarcoma, are less common. Feature suggestive of Lipoma is hyperechoic shadow, whilst cyst is indicated by well-defined anechoic shadow. Heterotopic pancreas is either hyperechoic or hypoechoic and granular in shape and sometimes can grow transmuraly. Tissues/fluid aspirated by EUS-guided puncture can be used for pathological and immunohistochemical examinations to establish the diagnosis.
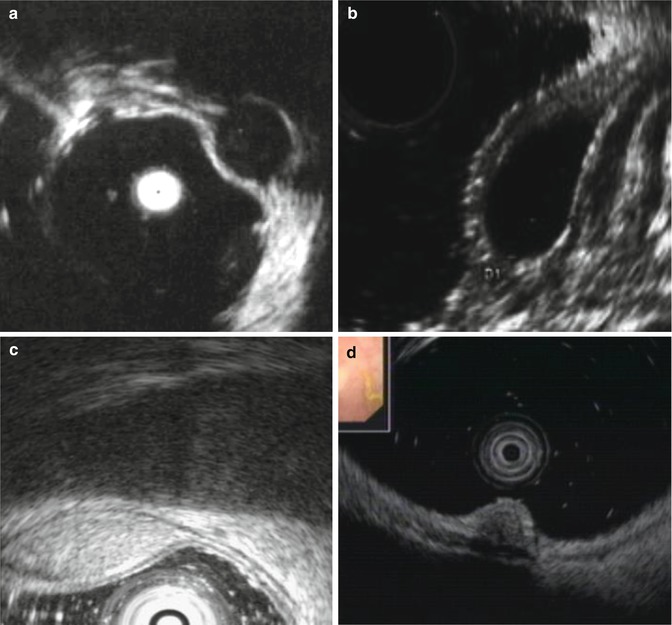

Fig. 1.4
Diagnosis of SMTs of large intestine by EUS. (a) Rectal stromal tumor or leiomyoma, derived from MP is hypoechoic; (b) Colonic cyst, derived from the submucosa is anechoic; (c) Colonic lipoma, derived from the submucosa is hyperechoic; (d) Rectal carcinoid, derived from the muscularis mucosa is heterogeneously echoic
EUS can determine the digestive tract wall layer from which the SMT is derived and this is critical in deciding suitability for endoscopic or surgical treatment. Over the recent years, by using the ESD technique we have successfully resected such various SMTs stromal tumor, leiomyoma, lipoma, glomus tumor, carcinoid, heterotopic pancreas and including tumors originating from MP.
1.1.3.3 Pancreato-Biliary System
Pancreas
EUS probe can be inserted through endoscope into duodenum to obtain ultrasonic image of the duodenal wall, duodenal papilla and periampulla. Five-layers of the duodenal wall can easily visualized that help to characterize whether a tumor is derived from the duodenal wall or periampullary area. Intraductal EUS (IDEUS) can be directly inserted into the pancreatic duct through the duodenal papilla to obtain clear images of the pancreas and peripancreatic area.
EUS is more sensitive than other imaging methods in assessing pancreatic tumors <2 cm in size. The diagnostic yield of pancreatic parenchymal tumors location and EUS guided fine needle aspiration (FNA) is relatively high. The sensitivity of EUS (98 % [95 % CI, 91–100 %]) for detecting a pancreatic mass is greater than that of CT (86 % [CI, 77–93 %]; P = 0.012). For the 53 surgical patients, endoscopic ultrasonography was superior to CT for tumor staging accuracy (67 % vs. 41 %; P < 0.001) but equivalent for nodal staging accuracy [1].
Pancreatic endocrine tumors can be either single or multiple with special biological behaviors. Most common examples are pancreatic islet cell tumors and ulcerogenic islet tumors. Generally, a pancreatic endocrine tumor can be detected when it is very small in size and over 90 % of them are smaller than 2 cm in diameter and well-defined with a hypoechoic structure. Endoscopic ultrasonography (EUS) offers a higher sensitivity (93–100 %) for detection of small potentially curable pancreatic masses when compared with existing imaging modalities [2].
EUS is relatively accurate in diagnosing chronic pancreatitis, comparable with ERCP, but better than CT scan. EUS can also give additional information such pancreatic stones and pancreatic duct dilation that may be accompanied by pancreas atrophy, local pancreatic enlargement or pseudocysts.
Biliary Tract Diseases
EUS is better that traditional abdominal US in obtaining higher resolution images because between the EUS probe and biliary system there is only a digestive tract wall.
EUS is accurate in diagnosing small tumors of the biliary system and enables assessment of depth of tumor infiltration. In addition detection of the adjacent lymph nodes by EUS in combination with the EUS-guided FNAB is high. In the assessment of bile duct cancer, it also provides additional staging information, whether portal vein, hepatic artery, head of pancreas and duodenal wall are invaded by the tumor. A miniature ultrasonic probe can be inserted into the bile duct that allows accurate diagnosis (early vs progressive) of the proximal bile duct tumors with high sensitivity and specificity. Preoperative and postoperative intraductal ultrasonography can assess bile duct cancer response to intraductal irradiation.
EUS is accurate to assess the depth of bile duct cancer infiltration. Traditional abdominal ultrasonography visualizes the gall bladder wall to a homogeneous and thin one-layered structure, while EUS visualizes the gall bladder wall to a three-layered structure by scanning through the duodenal bulb. Malignant lesions of the gall bladder wall are usually accompanied by the interruption or destruction of the continuous structure of the gall bladder wall, so the depth of infiltration by malignant lesions and whether such lesions have invaded into the liver can be determined by EUS.
EUS and IDEUS can clearly visualize the distal common bile duct and its contents hence has the advantage of diagnosing concurrent choledocholithiasis if present. Endoscopic ultrasonography has been proved to be of great sensitivity (up to 97 %) in the diagnosis of tiny stones that can be easily masked by contrast medium during ERCP, without any procedure-related complications and with a negative predictive value reaching 100 % [3].
1.1.3.4 EUS-Guided Fine Needle Aspiration Biopsy
EUS-guided fine needle aspiration (EUS-guided FNA) refers to a procedure in which a dedicated puncture needle is accurately advanced to the target under the real-time imaging and guidance by the ultrasonic probe mounted at the end of the ultrasonic endoscope and then fine needle aspiration of lesions is performed to conduct the pathological examination. Currently, the EUS-guided FNA technique has been applied in the diagnosis and treatment of lesions from areas such as gastrointestinal tract, mediastinum, lung, etc.
There are two types of EUS probes commonly used for puncture: linear array scan probes and rotating sector scan probes. The most commonly used probe is of the linear array type, which scans in a direction parallel to the needle route and can clearly visualize the needle route. In clinical application, different types of ultrasonic endoscopes are selected depending on the treatment purposes.
Role of EUS-FNA in Diagnosing SMTs
A SMT refers to a lesion between the deep mucosa and deeper serosa, more exactly, should be called “subepithelial lesion”. After a suspicious SMT is found, the nature of that SMT should be further characterized. EUS can make a definitive diagnosis of a typical lipoma, and cyst, but cannot offer tentative diagnosis in the case of a hypoechoic lesions. In such cases EUS-FNA deep biopsy usually is required to confirm the diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








