Histological types
Markers
βHCG
AFP
Choriocarcinoma
+++100 %
No
Pure seminoma
+ 10 %
No
Yolk sac tumour
No
++
Embryonal carcinoma
No
++
Pure teratoma
No
No
One must be cautious when interpreting these markers (AFP, βHCG and LDH) not only because of their lack of specificity, but also because of their limited sensitivity. A Spanish study has shown that markers are only detected in approximately 64 % of men with testicular cancer, while they remain ‘normal’ in about 38 % of men with disease recurrence [9].
Other markers: Placental alkaline phosphatase (PLAP) has been known for over 30 years as a marker for seminoma [10]. However, the usefulness of serum PLAP assay has been questioned when used alone and this marker is not widely available [11]. Cytogenetic and molecular markers (immunohistochemistry of c-kit, OCT 3/4 and determination of gain of chromosome 12, high mobility group proteins HMGA1 and HMGA2, SOX17, CDK10) are presently only utilized in research studies in specialized centres [12].
Staging by the serum markers: The use of staging by post-orchiectomy serum marker has been standardized and carries an important prognostic value (Table 20.2) [13]. Following orchiectomy, the time interval repeating measurements depends on the marker’s half-life. In practical terms a 6-week period is sufficient for both AFP and βHCG to normalize. A high value persisting beyond 6 weeks should raise suspicion of distant micro or macroscopic disease.
S (serum markers) staging | βHCG (mIU/ml) | AFP (ng/ml) | LDH (IU/L) |
|---|---|---|---|
SX | Marker studies not available or not performed | ||
S0 | Normal | Normal | Normal (190–390 IU/L) |
S1 | <5,000 | <1,000 | <1.5 × n (n = upper limit of the normal range) |
S2 | 5,000–50,000 | 1,000–10,000 | 1.5–10 × n |
S3 | >50,000 | >10,000 | >10 × n |
Possible causes of false positive results are marijuana abuse, hypoandrogenemia with consecutively raised LH and BHCG, liver diseases, gastric, pancreatic or lung carcinomas, ataxia-telangiectasia syndrome and familial AFP persistence [14]. Finally as for all statistical data, one should remember that the calculated normal values are based on a 95 % confidence interval, which means that up to 5 % of healthy men may be found to have a raised AFP.
For all these reasons, a moderately raised AFP >10 ug/L after surgery which remains stable without chemo- or radiotherapy may be compatible with clinical remission. Conversely, normal levels do not mean tumour-free status [9].
20.2.2 Other Laboratory Investigations
Blood tests should also be done for haemoglobin and WBC when infection is suspected. The presence of a large retroperitoneal mass with associated symptoms of abdominal colicky pain should instigate investigations to assess the high possibility of obstructive uropathy, that is, the renal function tests. This is also important before and during chemotherapy in view of the nephrotoxic potential of the drugs used, especially cisplatin.
Liver function tests and bone profile may be deranged in case of liver or bony metastases, respectively. Moreover, as noted above, liver dysfunction may cause a high AFP and it may be difficult for the clinician to recognize this as a false positive result during the investigations or follow-up of GCTs.
Semen analysis and hormonal profile should also be performed before orchidectomy and the initiation of chemotherapy.
20.3 Radiological Procedures
20.3.1 Primary Disease
Imaging studies are used to confirm the presence of intratesticular mass, to exclude paratesticular tumours and to evaluate the contralateral testicle for any significant lesions. Scrotal ultrasound is the radiological investigation of choice in assessing testicular pathologies. It is fast, inexpensive and easily available in health services. In addition, it is accurate with about 100 % sensitivity [15, 16].
Scrotal ultrasound also allows surveillance of the contralateral testis where it may detect microlithiasis which is associated with an increased risk of subsequent testicular cancer in the follow-up [17]. Testicular microlithiasis is categorized as follows: grade 1: 5–10 microliths/image, grade 2: 10–20 microliths/image and grade 3: >20 microliths/image. Grade 3 is regarded by some expert panels as most prone to subsequent GCT development [18]; however, other authors have shown that this grading has no effect on the prevalence of associated testicular tumours [19]. MRI is an excellent tool for soft tissue masses. It gives detailed information about the tumour and its extension. Compared to ultrasound, MRI is more specific but does not add much to clinical applications or decisions with regard to scrotal pathologies.
20.3.2 Disease Extension Studies
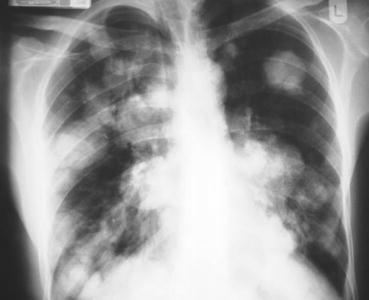
If not contraindicated, chest, abdomen and pelvic CT scan with contrast is the standard imaging means for disease–extension evaluation. It is very useful in assessing the retroperitoneum and the mediastinum for staging of the testicular GCT. However, it harbours a false negative rate of 20–30 % in GCT. When using CT scan, the threshold of metastatic lymph node detection is 8 mm diameter [20, 21]. The efficacy of abdominal MRI is equal to CT scanning in detecting retroperitoneal metastases. However, being more expensive and less widely available, MRI is only used when CT scan is contraindicated. CT scan of the head is recommended when there is clinical suspicion of brain secondaries or in cases of choriocarcinoma due to its high possibility of haematogenous spread. A simple chest X-ray in the metastatic workup may be sufficient in some cases to detect chest secondary lesions (Fig. 20.1), but a CT scan must be performed to confirm any suspicious finding on X-ray or in cases of a bulky tumour. When CT scan has revealed retroperitoneal residual masses after chemotherapy for seminoma, further evaluation can be performed by a FDG–PET scan to assess hypermetabolic or necrotic lesions [22]. The presence of viable tissue among the residual tumours is an indication for retroperitoneal lymph nodes dissection (RPLND) (see pictures). It is important to note that teratomas do not show hyperactivity in a FDG–PET scan; therefore, the presence of a viable carcinoma or mature teratoma cannot be differentiated from necrosis or fibrosis. This fact was histologically confirmed by a prospective German trial which showed FDG-PET sensitivity and specificity of 70 % and 48 %, respectively, in detecting NSGCTs. FDG-PET was unable to provide clear additional clinical benefit compared to the standard diagnostic means with positive predictive values of 59, 55 and 61 % for FDG-PET, CT and serum markers, respectively [23].
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






