Fig. 2.1
Preoperative nomogram for prediction of organ-confined stage of the UTUC (adapted from Margulis V, et al.: Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol. 184: 453-8, 2010)
Several different sequences are needed to improve performance. Thus, it is possible to obtain reconstructions of urinary tracts comparable to IVU sequences with TSE (turbo spin-echo), HASTE (T2-weighted apnea), and MIP (3D T1-weighted gadolinium and furosemide). The MR urography has a lower spatial resolution and more artifacts by hematuria or the presence of a ureteral catheter than CT urography. Although only a few results have been published on this subject, the detection of UTUCs with MR urography has been demonstrated with a sensitivity of 70–80 % and specificity close to 100 % [10].
More recently, DWI (diffusion-weighted images) with calculation of apparent diffusion coefficient (ADC) was evaluated in the diagnosis of UTUC [11]. This technique, which does not require injection of gadolinium, is based on the diffusion of water molecules in tissue. This allows the calculation of the ADC for the renal parenchyma, dilated urinary system, and tumor. Several studies agree that the ADC of the pelvicaliceal cavities UTUC is significantly lower that of the adjacent tissue, making this sequence of interest for diagnosis in addition to conventional sequences [12]. The value of ADC in tumor staging is currently limited with conflicting data in the literature but appears to be associated with the tumor grade and proliferation markers [12, 13].
As with CT urography, MR urography has limited accuracy in staging cTa-cT2 grade tumors [14].
Endoscopy
Retrograde Ureteropyelography
Retrograde ureteropyelography (RUP) is usually performed during cystoscopy and/or flexible ureteroscopy under general anesthesia and is therefore not a diagnostic modality in its own right. This procedure consists of retrograde opacification by prior catheterization of the upper urinary tract with a ureteral catheter.
Using this approach, the delineation of the upper urinary tract following opacification demonstrates performance comparable to CT urography in terms of diagnostic sensitivity and specificity (96 % and 97 %, respectively) [15]. However, there is a bias since many patients already have a suspected lesion prior to going to the operating room for a RUP. This examination is recommended by several guidelines including that of the EAU (grade C) [2] and AUA.
Flexible Ureteroscopy and In Situ Biopsies
Technological advances have made ureteroscopy a valuable diagnostic tool for the evaluation of suspicious lesions in the upper urinary tract. Indeed, the use of flexible devices enables the complete examination of the upper tract, including the lower pole renal calices.
Ureteroscopy is particularly useful in cases of uncertain diagnoses, in cases of patients with a solitary kidney, or when conservative treatment is considered. Ureteroscopic examination is now recommended by the guidelines for the first-line screening of UTUC (recommendation of grade C) [2].
Flexible ureteroscopy allows a visual diagnosis in 95 % of cases [2]. Advances in image resolution with digital ureteroscopes reinforce the quality of this examination. The detection sensitivity could be improved by photodiagnosis using 5-aminolevulinic acid (5-ALA), especially for small lesions and carcinoma in situ, or Narrow Band Imaging (NBI) [16, 17]. The NBI system uses only a part of the visible spectra (between 415 and 540 nm), which enhances the contrast of the tumor neovascularization and thus the detection of the small lesion. The preliminary results are promising, with 22.7 % more lesions detected using this technique than with white light [17]. Further studies are warranted to evaluate whether tangential viewing within the ureter poses a limitation towards the use of these new technologies within the upper tract.
Morel et al. showed the limited applicability of rigid ureteroscopy in this context due to inability to explore the pyelocaliceal cavities [18].
However, the macroscopic visual diagnosis does not allow the infiltrating nature of the tumor to be determined. According to El-Hakim et al., the endoscopic appearance of tumors leads to errors of staging in at least 30 % of cases [19].
During the procedure, the surgeon can perform selective cytology and biopsy of a visualized suspicious lesion in addition to the visual macroscopic diagnosis. This pathologic biopsy can confirm the diagnosis with a sensitivity of 89–95 % and obtain a prognostic tumor grade and stage [20]. The reliability of biopsies for tumor staging is poor, with 45 % of tumors classified as Ta, but these are actually more invasive tumors. However, the biopsy grade is adequately correlated with the final histopathological grade in 69–91 % of cases and the final tumor stage. Often the grade of the tumor gives information about the likely stage. In fact, the detection of a grade 1 tumor on biopsy corresponds to a noninvasive tumor (≤pT1) in 68–100 % and the detection of a grade 3 tumor on biopsy corresponds to an invasive tumor (≥pT2) in 62–100 % cases [21–24]. Upper tract ureteroscopy may not identify any tumor up to 50 % of the time even in patients with positive urine cytology.
However, obtaining biopsies is not always possible. Indeed, the introduction of biopsy forceps through the working channel of the ureteroscope may limit deflection and therefore the accessibility of the lower calyx.
The assessment of UTUC depth invasion remains a challenging task in ureteroscopy. The use of endoscopic ultrasonography has been described for this task but has not been routinely used in daily practice [25]. New imaging techniques such as optical coherence tomography (OCT) or endoscopic confocal microscopy are now in development and could eventually be used for this specific evaluation and as a complement to lesion biopsies [26, 27].
The hypothesis of the possible “seeding” of cancer cells during the procedure (by hyperpressure of the cavities or trauma to the wall) is not currently discussed [28]. The delay attributable to the performance of a diagnostic ureteroscopy with biopsy does not significantly affect the long-term prognosis of the disease [29].
Although rare, complications associated with diagnostic ureteroscopy are possible (0.5–5 % of cases). These consist of perforations, stripping of the ureter, stenosis, or parenchymal infections.
Cystoscopy
This simple test is recommended (grade A) as a first-line screening to rule out any synchronous bladder tumors.
Urine Cytology
Urine cytology is based on the analysis of cells exfoliated in urine and remains recommended for the diagnosis of UTUC (grade A) [2].
The cells can be obtained following urination or samples taken by bladder wash during cystoscopy or in situ during ureteroscopy. Although easy and noninvasive, voided urinary cytology is limited by its variable sensitivity (35–65 %) according to the large interindividual variability in the interpretation and the increased level of false positives in cases of trauma of urothelium or inflammation. The specificity of this technique is also excellent (>90 %).
In cases of positive urine cytology with normal cystoscopy, the likelihood of UTUC is very high. However, urine cytology is poor at predicting final tumor stage and grade. Messer et al. reported that positive urine cytology predicts a high-grade tumor with a sensitivity of 56 % and an invasive tumor with a sensitivity of 62 % [30]. Additionally, a positive preoperative urine cytology appears to be a risk factor for intravesical recurrence [31].
Predictive Tools
Currently, treatment decisions regarding UTUC are based on the clinician’s ability to predict the risk of progression based on the individual pathology of each patient.
Therefore, predictive and prognostic statistical tools have been developed to assist the clinician in predicting the progression risk of the disease.
The combination of the various elements previously described should increase the prediction performance of such tools.
Margulis et al. developed a nomogram based on clinical criteria (biopsy grade, tumor architecture, and location) to estimate the probability of locally advanced disease [34]. Their tool showed an accuracy of 76.7 %.
Similarly, Favaretto et al. developed a tool to predict the invasiveness or locally advanced nature of UTUC based on the criteria of imaging data and ureteroscopy, which includes biopsy grade [35]. This tool predicts the infiltrating character with 71 % accuracy and the locally advanced nature with 70 % accuracy.
Currently, no molecular parameter has yet been incorporated into these nomograms, unlike with other pathologies such as colon cancer (K-ras status) and breast cancer (HER2 status).
These tools could be helpful for selected patients who require radical treatment and/or extensive lymphadenectomy and/or neoadjuvant chemotherapy; however, independent validation of these tools is required today as they are not currently used in clinical practice, external validation is not yet available, and these tools are not yet used in clinical practice.
We now propose the flowchart in Fig. 2.2 to summarize this chapter.
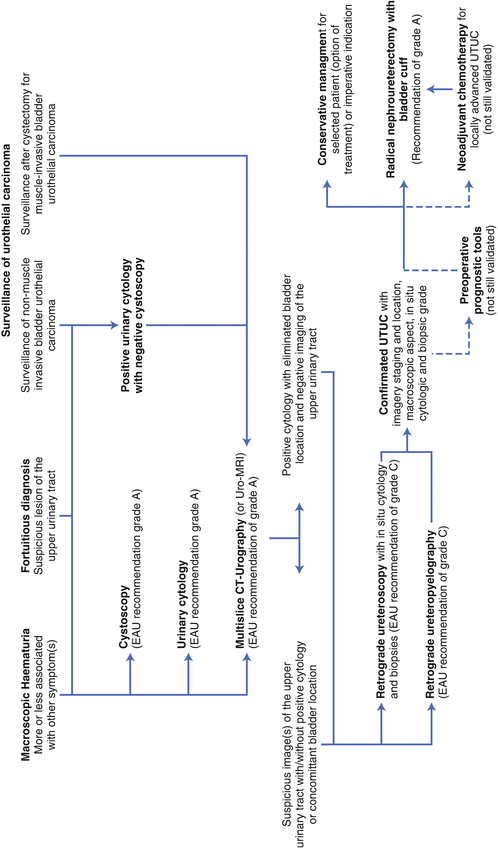

Fig. 2.2
Flowchart summarizing the diagnosis and evaluation of UTUC
Conclusion
Due to recent advances in imaging and endoscopy, the diagnostic management of UTUC has evolved in recent years. CT urography is now the tool for the detection of UTUC. Flexible ureteroscopy is often needed for the pretreatment patient evaluation. Future developments are expected to further refine the preoperative staging of these tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






