Gastroparesis is a complication of long-standing type 1 and type 2 diabetes mellitus. Symptoms associated with gastroparesis include early satiety, prolonged postprandial fullness, bloating, nausea and vomiting, and abdominal pain. Mortality is increased in patients with diabetic gastroparesis. A subset of patients with diabetic gastroparesis have pylorospasm that results in obstructive gastroparesis. Current treatment approaches include improving glucose control with insulin and prescribing antinauseant drugs, prokinetic agents, and gastric electric stimulation. Future directions include improved diet counseling based on gastric emptying rate, continuous insulin delivery systems with glucose sensor-augmented monitoring, and drugs for correcting gastric neural and electric abnormalities.
Key points
- •
Gastroparesis is delayed gastric emptying in the absence of obstruction, a complication that affects patients with type 2 as well as type 1 diabetes mellitus.
- •
Symptoms associated with gastroparesis are nonspecific, and the diagnoses should be confirmed with gastric emptying tests.
- •
Patients are often overweight and have nutritional deficiencies.
- •
Obstructive gastroparesis, a subset of gastroparesis, is caused by pyloric dysfunction, and botulinum toxin A injections may be helpful.
- •
Trending postprandial glucose excursions with continuous glucose monitoring aids in the dosing and timing of insulin administration in diabetic patients with gastroparesis.
Introduction
When gastroparesis afflicts patients with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM), the consequences are particularly severe. Symptoms associated with gastroparesis, such as early satiety, prolonged fullness, nausea, and vomiting of undigested food, not only reduce the quality of life but also compound difficulties in controlling blood glucose levels.
Gastroparesis is defined as a delay in the emptying of ingested food in the absence of mechanical obstruction of the stomach or duodenum. Many patients with diabetes (as well as their physicians) do not appreciate that gastroparesis has developed. In diabetic patients with gastroparesis, ingested food is not emptied in a predictable period of time; thus, the anticipated nutrient absorption is not the reality. Consequently, the selected dose and timing of insulin therapy to control postprandial glucose may be inappropriate.
In many patients with gastroparesis, erratic postcibal glucose levels result in swings from hypoglycemia to severe hyperglycemia and even ketoacidosis. Hyperglycemia itself elicits gastric dysrhythmias and slows gastric emptying. Patients frequently are seen in emergency rooms for low glucose levels, severe hyperglycemia, or ketoacidosis. Gastroparesis as an underlying condition needs to be considered in these cases.
In addition to antinauseant and prokinetic drug therapies, patients with diabetic gastroparesis also need to change their diet and the timing and dosing of insulin to better match the slow emptying of ingested food. The epidemiology, pathophysiology, clinical presentation, diagnostic testing, and treatments for diabetic gastroparesis are reviewed in this article.
Introduction
When gastroparesis afflicts patients with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM), the consequences are particularly severe. Symptoms associated with gastroparesis, such as early satiety, prolonged fullness, nausea, and vomiting of undigested food, not only reduce the quality of life but also compound difficulties in controlling blood glucose levels.
Gastroparesis is defined as a delay in the emptying of ingested food in the absence of mechanical obstruction of the stomach or duodenum. Many patients with diabetes (as well as their physicians) do not appreciate that gastroparesis has developed. In diabetic patients with gastroparesis, ingested food is not emptied in a predictable period of time; thus, the anticipated nutrient absorption is not the reality. Consequently, the selected dose and timing of insulin therapy to control postprandial glucose may be inappropriate.
In many patients with gastroparesis, erratic postcibal glucose levels result in swings from hypoglycemia to severe hyperglycemia and even ketoacidosis. Hyperglycemia itself elicits gastric dysrhythmias and slows gastric emptying. Patients frequently are seen in emergency rooms for low glucose levels, severe hyperglycemia, or ketoacidosis. Gastroparesis as an underlying condition needs to be considered in these cases.
In addition to antinauseant and prokinetic drug therapies, patients with diabetic gastroparesis also need to change their diet and the timing and dosing of insulin to better match the slow emptying of ingested food. The epidemiology, pathophysiology, clinical presentation, diagnostic testing, and treatments for diabetic gastroparesis are reviewed in this article.
Epidemiology
A recent update reported that there are more than 36 million individuals with diabetes in North America and the Caribbean and most are cases of T2DM. The estimates of prevalence of gastroparesis in T1DM vary widely. Although in tertiary centers, up to 40% of patients with T1DM have gastroparesis, surveys in Olmsted County, Minnesota, indicated a prevalence of 5%.
Similarly, in specialized centers, 10% to 30% of patients with T2DM have gastroparesis ; in Olmsted County, the prevalence was 1%. These differences likely reflect a selection bias, because more patients with diabetes and complications are seen in tertiary medical centers compared with surveys of patients in the community. Nevertheless, because of the increasing numbers of patients with T2DM, this population represents the largest group of patients with gastroparesis.
The number of patients with diabetes worldwide continues to increase. The World Health Organization estimated that in 2013 almost 350 million individuals had diabetes (mainly T2DM), and predicted mortality from diabetes will double by 2030 ( http://www.who.int/mediacentre/factsheets/fs312/es/ ). Assuming a low estimate of gastroparesis incidence in T2DM of 1%, at least 5 million individuals with diabetes complicated with gastroparesis will require specialized diagnosis and care.
Gastroparesis evolves over time, presumably as acute and chronic hyperglycemia and reduced insulin and insulinlike growth factor 1 (IGF-1) signaling results in damage to the interstitial cells of Cajal (ICCs) and enteric neurons of the stomach. Over a 10-year period, approximately 5.2% of patients with T1DM developed gastroparesis, whereas 5 times fewer (1%) patients with T2DM developed gastroparesis over that same period. Although good control of glycemia prevents or delays many of the chronic complications of T1DM, the effect of good glucose control on the onset or progression of gastroparesis in T1DM is unknown. Diabetic patients with gastroparesis often have many of the chronic complications of diabetes (retinopathy, nephropathy) and increased hospital use. In a few patients, gastroparesis is the first diabetic, neuropathic complication.
Compared with T2DM, patients with T1DM with gastroparesis are younger, thinner, and tend to have more severe delays in gastric emptying. Mortality is increased in diabetic patients when they develop gastroparesis and is usually related to cardiovascular events when compared with diabetic patients without gastroparesis.
Normal postprandial gastric neuromuscular activity
The normal stomach performs a series of complex neuromuscular activities in response to the ingestion of solid foods. First, the fundus relaxes to accommodate the volume of ingested food ( Fig. 1 ). Normal fundic relaxation requires an intact vagus nerve and is mediated by enteric neurons containing nitric oxide. The relaxation of the fundus allows food to be accommodated without excess stretch on the fundic walls.

Second, the corpus and antrum produce recurrent peristaltic waves that mix or triturate the ingested solids into fine particles termed chyme. The waves mix together the food particles, pepsin, and acid to prepare the ingested food for emptying. Peristaltic waves in the corpus-antrum occur at a frequency of 3 contractions per minute, a frequency that is dictated by the gastric pacemaker cells (the ICCs), which normally depolarize and repolarize at a rate of 3 cycles per minute (cpm) (see Fig. 1 ; Fig. 2 ). The slow waves (pacesetter potentials) originate at the greater curvature of the stomach between the fundus and proximal corpus (see Fig. 2 ) and migrate in a circumferential and aboral direction at increasing velocity in the distal antrum. The slow waves bring the circular muscle of the stomach to depolarization threshold and contractions, which occur in response to the release of acetylcholine. The action and plateau potentials are synchronized to the 3-cpm slow wave, thus resulting in the coordinated 3-per-minute peristaltic contractions.
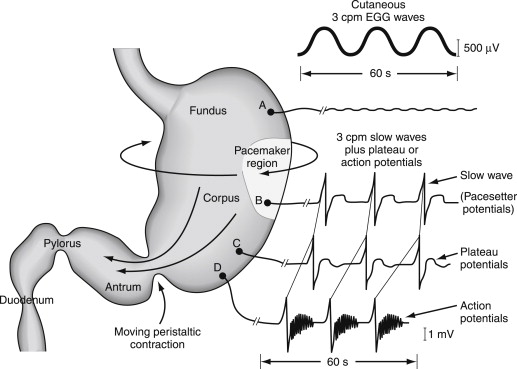
Third, emptying of chyme contents begins when the ingested solid foods are sufficiently triturated. The peristaltic waves at 3 per minute empty aliquots of chyme through the pylorus into the duodenum (see Fig. 1 ). The pylorus acts as a sieve and can regulate the particle size as well as the volume of chyme that is emptied into the duodenum with each peristaltic wave. In the normal condition, the number of calories emptied per minute is consistent, at about 5 calories per minute in humans. The emptying of food from the stomach is altered by the nature of the constituents (carbohydrate, protein, and fat) and the fiber and indigestible components. Carbohydrates are emptied faster than proteins, which are emptied faster than fats, which delay gastric emptying. Soluble and insoluble fibers are emptied after the nutrients. Gastric emptying is also regulated by postpyloric influences. The release of cholecystokinin slows gastric emptying. Intraluminal content with high concentration stimulates the release of peptide YY from the ileum to slow gastric emptying. Normal postprandial neuromuscular activity is associated with a sense of comfortable fullness. In contrast, the ingestion of food elicits early satiety, nausea, and epigastric discomfort or pain in diabetic patients with gastroparesis.
Pathophysiology of diabetic gastroparesis
Gastric Neuropathy and Cajalopathy in Diabetic Gastroparesis
Full-thickness biopsies of the gastric corpus from patients with T1DM and T2DM and gastroparesis indicate that the disease is primarily a disease of gastric enteric neurons and ICCs. We know that ICCs are depleted (<5/hpf compared with controls) in the diabetic gastroparesis stomach. Gastric enteric neurons are decreased in numbers of cell bodies and processes are truncated. These neurons are surrounded by an immune infiltrate composed primarily of type 2 macrophages, suggesting a role for the immune system and carbon monoxide in the pathogenesis of diabetic gastroparesis. The circular and longitudinal smooth muscle layers are normal or have very mild fibrosis. ICCs are depleted in diabetic mice with gastric emptying abnormalities. Hyperglycemia in these animals is associated with dedifferentiation of ICCs into immature myoblasts, and intense insulin therapy restores ICC numbers to normal. It is postulated that platelet-derived growth factor (+) myoblasts have the potential to evolve into ICCs.
Abnormalities of Fundic Relaxation
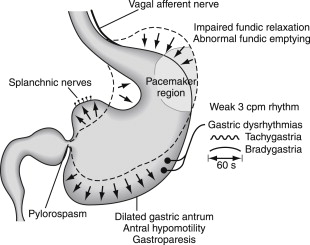
Relaxation of the fundus during ingestion of food requires normal vagus nerve function and the release of nitric oxide from inhibitory neurons. In patients with diabetes, the fundus fails to relax normally ( Fig. 3 ). The ICCs function also as stretch receptors. The loss of nitrergic neurons plus the absence of ICCs may account for the poor fundic relaxation and decreased gastric capacity seen in gastroparesis.
Disorders of the Corpus-Antrum
The corpus and antrum perform the mixing and emptying activities of the stomach. In diabetic gastroparesis, corpus-antral contractions are ineffective, although the smooth muscle layers seem to be normal. Thus, the depletion of ICCs and presence of abnormal enteric neurons are the mechanisms of gastric neuromuscular dysfunction. Loss of enteric neurons results in less acetylcholine release for contractions and less nitric oxide for relaxation of smooth muscle. Depletion of ICCs is associated with the presence of gastric dysrhythmias and loss of the normal 3-cpm myoelectric rhythm. Gastric dysrhythmias range from tachygastrias to bradygastrias and a variety of aberrant conduction pathways in the corpus-antrum. Gastric dysrhythmias reduce efficiency and the occurrence of normal gastric peristaltic waves and, thus, lead to slow gastric emptying and gastroparesis (see Fig. 3 ). Correction of gastric dysrhythmias with domperidone, a peripheral dopamine 2 antagonist, improved upper gastrointestinal (GI) symptoms, suggesting dysrhythmias correlate with symptoms.
Disorders of Pyloric Relaxation
The pyloric sphincter also regulates gastric emptying. The pylorus provides resistance to flow and a sieving function for particles as antral peristaltic waves propel chyme from the antrum into the duodenum (see Fig. 1 ). Relaxation of the pyloric sphincter to allow flow is mediated by nitric oxide released from enteric neurons. In a subset of patients with idiopathic and diabetic gastroparesis, pylorospasm (failure of pyloric relaxation in coordination with antral peristaltic waves) results in gastroparesis (see Fig. 3 ). Mechanical obstruction at the pylorus or post bulbar duodenum caused by ulcer disease or cancer must be excluded in patients with gastroparesis.
Clinical Presentation
Symptoms associated with diabetic gastroparesis are early satiety, prolonged fullness, bloating, nausea and vomiting, and abdominal discomfort and pain. These symptoms are vague and nonspecific. Approximately 20% of patients develop these symptoms acutely and with a febrile illness. A variety of diseases may cause these symptoms, and abdominal pain and causes of symptoms other than gastroparesis must be considered.
Nausea is the most bothersome and predominant symptom in diabetic patients with gastroparesis. Nevertheless, the nausea caused by gastroesophageal reflux disease (GERD) or constipation or gallbladder disease, common disorders in patients with diabetes, must be considered. Nausea related to gastroparesis is typically located in the epigastrium and usually increases in severity after ingestion of meals. Vomitus contains chewed food. Prolonged stomach fullness and vague epigastric discomfort are common. Symptoms are similar in patients with T1DM and T2DM, although patients with T2DM tend to have more fullness and bloating. Table 1 lists demographic parameters and symptoms in patients with T1DM and T2DM and gastroparesis. In contrast to patients with idiopathic gastroparesis, fewer diabetic patients with gastroparesis report pain as a predominant symptom.
| T1DM (n = 78) | T2DM (n = 59) | P Value | |
|---|---|---|---|
| Female (%) | 70 | 76 | |
| Age (y) | 39 ± 11 | 53 ± 11 | P <.001 |
| Married (%) | 54 | 64 | |
| Ever smoked (%) | 29 | 39 | |
| Time from diabetes mellitus onset to initial symptom (y) | 14 ± 11 | 8.4 ± 8 | P <.005 |
| Symptom duration (y) | 6.2 ± 6 | 4.1 ± 3 | |
| BMI | 26 ± 6 | 33 ± 8 | P <.001 |
| Normal BMI (%) | 47 | 14 | |
| HbA 1c | 8.3 ± 2 | 7.4 ± 1.7 | P <.003 |
| Major depression (%) | 28 | 32 | |
| GCSI | 2.8 ± 1.1 | 3.0 ± 1.0 | |
| GET at 4 h (%) | 47 ± 27 | 33 ± 24 | P <.001 |
| Severe GET (%) | 54 | 32 | P <.001 |
| Number of hospitalizations in past year | 5.1 ± 6.4 | 3.2 ± 6.6 | P <.003 |
In some patients (20%) with gastroparesis, abdominal pain is the predominant symptom. The pain should be evaluated separately from other symptoms associated with gastroparesis in an attempt to determine a specific cause for the pain. Chronic cholecystitis, peptic ulcer diseases, and the abdominal wall syndrome need to be excluded. Stomach pain can be caused by pylorospasm or gastric sensitivity to stretch in patients with gastroparesis. Mechanical obstruction at the pylorus caused by ulcer or cancer must be excluded in patients with gastroparesis.
Physical examination may be normal or show obesity or undernutrition, retinopathy, neuropathy, or vitamin deficiency (cheilosis). Obesity in patients with T2DM is a risk factor for gastroparesis. Abdominal examination may show distension, a succession splash, or positive Carnett sign. A positive Carnett sign indicates that abdominal pain is from an abdominal wall syndrome secondary to nerve entrapment or inflammation, often located at a healed incision site.
Standard laboratory studies are usually normal. Hemoglobin A 1c levels have a wide range. Thyroid-stimulating hormone levels and fasting cortisol should be measured to screen for Addison disease and hypothyroidism. Vitamin D levels are frequently low.
Tests for gastroparesis and gastric dysrhythmias
Solid-Phase Gastric Emptying Test
Tests for gastroparesis and gastric dysrhythmias are nuclear medicine scintigraphy, wireless capsule endoscopy, and electrogastrography (EGG). These tests should be performed after upper endoscopy to rule out mechanical obstruction, which produces symptoms similar to gastroparesis. The most standardized test for gastric emptying is the technetium-labeled low-fat egg albumin-based meal. The patient must stop prokinetic agents 7 days before the test, fast after midnight, and blood glucose level on the day of the test should be less than 270 mg/dL. Immediately after the patient ingests the 257-calorie meal, a 1-minute scintigram is obtained with the patient in a sitting position and then for 1 minute every hour for 4 hours. Normal gastric emptying is 39% or less of the meal retained at 2 hours and 9% or less retained at 4 hours. Thus, gastroparesis is diagnosed by a documented retention of 40% or more at 2 hours or 10% or more at 4 hours.
The solid-phase gastric emptying test is also important, because some patients who have the symptoms associated with gastroparesis have rapid gastric emptying or dumping syndrome. In dumping syndrome, less than 30% of the test meal is retained at 60 minutes.
Wireless Capsule Motility Test
The wireless capsule motility test measures intraluminal pH and pressure. The capsule is swallowed during ingestion of a nutrient bar that contains the same number of calories as the Egg Beaters test meal. No further food intake is allowed for 5 hours. If the capsule does not empty from the stomach into the duodenum in 5 hours, then delayed gastric emptying is diagnosed. Small bowel and colon transit time are also measured, and results may help in determining the underlying pathophysiology of other GI symptoms.
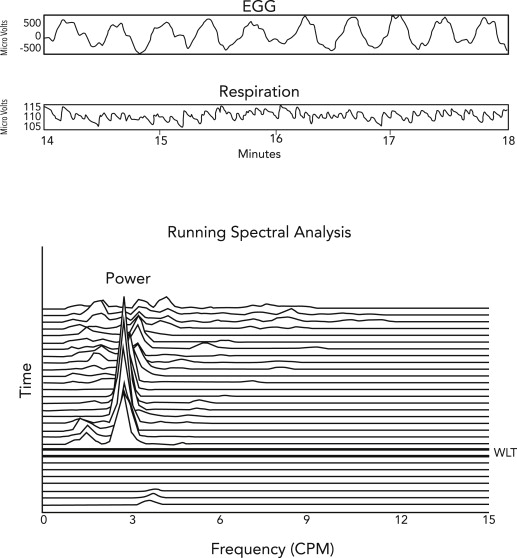
Electrogastrography
Electrogastrography is the method of recording gastric myoelectric activity with a noninvasive method. Electrocardiography-type electrodes are placed on the epigastrium, and the myoelectric signal is recorded before and after a water load or a nutrient load test. Normal gastric myoelectric activity (2.5–3.7 cpm) normally increases after the water load test. Gastric dysrhythmias are defined as tachygastrias (3.5–10 cpm) or bradygastrias (1–2.5 cpm). Tachygastrias and bradygastrias are associated with loss of ICCs; on the other hand, a normal 3-cpm rhythm is associated with the presence of normal numbers of ICCs. A subset of patients with gastroparesis has normal or increased 3-cpm electric activity, a discordant finding that indicates the possibility of obstructive gastroparesis secondary to pyloric stenosis or pylorospasm ( Fig. 4 ).