Fig. 1
Characterization of the DC kidney scaffolds. (a) Gross appearance of harvested DC kidney scaffolds. (b) Electron microscopy observation shows intact extracellular matrix in DC kidney scaffold. Blue arrows indicate the membrane of Bowman’s capsule, a green arrow indicates the basement membrane of the glomerular capillaries, and a red star points to the mesangial matrix. (c and d) Vascular corrosion casting shows a normal vascular tree of DC kidney scaffold (d) compared with intact kidney (c). (e and f) H&E staining shows the existence of blue-stained nuclei in intact kidney (e) but not DC kidney scaffold (f). (g) Masson’s staining shows that green-stained collagenous fibers in DC kidney scaffold. (h) PAS staining shows the presence of the ECM (e.g., basement membranes) in DC kidney scaffold. Note that the capillary loops of the glomeruli are clearly displayed. Scale bars = 5 mm (a, c, and d), 100 μm (e–h) and 5 μm (b) (Reproduced from YL Yu et al. Biomaterials 2014; 35: 6822–6828 with permission of Elsevier)
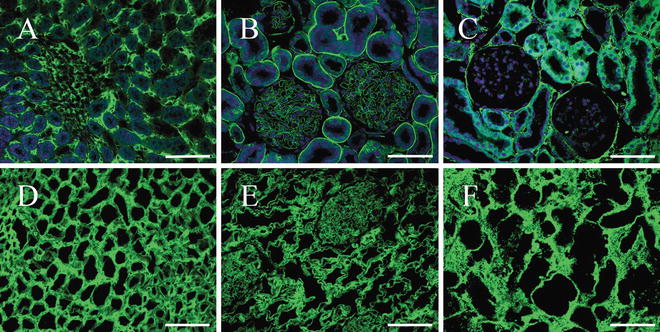
Fig. 2
Immunofluorescence of collagen IV, laminin (LN), and fibronectin (FN). (a–c) Native kidney. (d–f) Decellularized kidney scaffolds. (a and d) Scale bars = 100 μm. (b, c, e, and f) Scale bars = 50 μm. In all panels, cell nuclei stain was blue with DAPI, while fluorescent immunohistochemical staining for specific markers appears green. These indicate the intact kidney architecture and matrix proteins are retained and undisturbed, while cells and nuclear material are removed compared with the native
Given that SDS is toxic to cells, it is important to confirm the absence of SDS residues in the scaffolds. In our studies, the concentration of SDS residues in DC kidney scaffolds is typically 50.0 ± 1.7 μg/g, which is well below the toxic level of 133.3 μg/g.
4 Notes
1.
In the decellularization process, it is very important that the detergent used is sodium dodecyl sulfate, not sodium dodecyl sulfonate.
2.
For decellularization, we don’t use trypsin, as it will damage the structural proteins severely.
3.
Before cannulation, make sure that ligated vessels include the suprarenal vena cava, suprarenal abdominal aorta, lumbar arteries, testicular arteries of male, and ovarian arteries of female animals.
4.
A higher or lower flow velocity is not suitable.
5.
All of the fluids for decellularization should be preheated to 25–30 °C.
6.
After abdominal aorta cannulation, perfuse with heparin in PBS for 30 min to prevent blood clotting.
7.
The concentration of SDS and Triton X-100 must be checked, as elevated SDS and Triton X-100 will damage the extracellular matrix microstructure. Conversely lower detergent concentrations do not work well.
8.
Given that SDS is toxic to cells, it is necessary to ensure that there is sufficient perfusion of deionized water to remove all traces of SDS.
9.




The prepared DC scaffolds can be preserved for 1 week at 4 °C and 2 months at −80 °C.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







