Patient risk factors
Tobacco use
Alcohol use
Obesity
Age >65
ASA > 3
Malnutrition <3.0
Weight >10 % TBW
Disease factors
Emergent surgery
Steroid or immunomodulator use
Malignancy
Level of tumor (rectal lesion below peritoneal reflection
Anastomotic height
Preoperative radiation therapy
Intraoperative factors
Poor perfusion to conduit and anastomosis
Anastomosis or conduit under tension
Sigmoid colon conduit
Prolonged operative time
Excessive blood loss
Blood transfusion
Prevention of Anastomotic Leaks: Techniques and Adjuncts
Prevention and reduction of risk of anastomotic leaks requires recognition and mitigation of risk factors pre-operatively and intra-operatively as well as the use of adjuncts. Intra-operatively, meticulous technique to prevent excessive blood loss, undue tension on the anastomosis and ensuring a good blood supply are vital to ensuring a quality anastomosis. Multiple intraoperative and postoperative adjuncts are utilized with varying degrees of success. Intra-operative adjuncts include the use of buttressing materials, air leak testing the anastomosis and a restrictive resuscitation strategy. In the post-operative period, supplemental oxygen therapy, the use of protective diverting stomas and pelvic drains are also reported.
Intervening on modifiable patient risk factors such as obesity, smoking and alcohol use should be attempted, though not often feasible in the setting of malignancy where more prompt surgical intervention is performed. Patients with known pre-operative malnutrition should receive supplemental nutrition in the form of enteral nutrition or TPN prior to undertaking operative intervention. Preoperative prophylaxis in the form of bowel preparation or IV and PO antibiotics, while decreasing the rate of surgical site infections, has not been shown to decrease the rate of leak on colorectal anastomosis [68, 80], and is left to the discretion of the surgeon.
Intra-operatively, adequate perfusion to the conduit and anastomosis can be difficult to ascertain and clinical judgment is needed as there are no reliable or accurate devices that are widely available [52]. Indicators of perfusion include the color of the mucosa and bleeding from the staple line. The prevention of tension on the anastomosis will also ensure perfusion. This is achieved by high ligation of the inferior mesenteric vein at the level of the duodenum and artery pedicle. Although this can compromise blood supply, this is usually not typical when collateral circulation is intact and care is taken to avoid resection of the mesentery too close to the bowel. A bulldog or vascular clamp may be placed initially on the pedicle prior to formal ligation to temporarily stop blood flow and evaluate for collateral supply. Additionally, proper mobilization of the splenic flexure will assist in achieving adequate length for the proximal bowel to reach the pelvis. Sigmoid colon conduits are generally not recommended because of tenuous blood supply after high ligation. One of the most difficult anastomosis with regards to blood supply and tension occurs in the setting of preoperative XRT and the need for a colo-anal anastomosis. Meticulous technique in creation of the anastomosis cannot be overemphasized. The use of hand-sewn versus stapled technique has been extensively studied in the literature with the most recent Cochrane meta-analysis finding both techniques are equivalent [52, 81, 82]. Single versus double-layer sutures have also been studied with randomized data reporting no adverse outcomes in either suture group [83]. Yet, in mid-to-low pelvic anastomosis, the decision of which to perform is often a moot point, as stapled anastomoses are technically easier and are predominately performed.
Buttressing materials (i.e., fibrin glue, SEAMGUARD® (W.L. Gore & Associates, Newark, DE] along the staple line or omentoplasty have had mixed reports of success in preventing leaks [84–91]. The air leak test is supported throughout the literature as a simple method of determining the integrity of a fresh anastomosis prior to closure and helps identify any problems with the anastomosis that can be addressed intraoperatively or suggest proximal diversion may be required [92–95]. While clinical leaks may still develop, testing for air leaks is not known to be harmful, and positive tests have been associated with higher rates of subsequent clinical leaks [52]. Restrictive fluid strategy is reported in the literature to decrease overall post-operative complications, however its role in prevention of leak is less clear. Most studies demonstrate a good safety profile when compared to usual care and its use can be considered good practice [96–102].
Post-operative adjuncts include the use of supplemental oxygen, pelvic drains and a protective, diverting stoma. While most of the supplemental oxygen studies focus on the prevention of surgical site infections, there are several studies regarding the use of supplemental O2 in prevention of anastomotic leak [103, 104]. By increasing the O2 saturation in arterial blood, it is believed to increase the mucosal O2 tension at the site of bleeding and prevent ischemia. This was initially demonstrated in a rat model that demonstrated a higher bursting pressure and hydroxyproline content (a marker of collagen content) after hyperbaric chamber treatment [90]. Following this, a Spanish study used 80 % FiO2 in 45 patients undergoing LAR and found better tissue oxygen levels at the anastomosis compared to controls with no complications in either test group. In a recent randomized control trial, patients were randomized to a control arm of 30 % Fi02 or experimental arm of 80 % FiO2 for 6 h post rectal cancer resection. In this study, there was 46 % reduction in anastomotic complications (p < 0.05) [103].
Protective stomas are utilized to divert fecal flow away from the fresh anastomosis; however, there is ongoing debate as to whether or not the presence of a diverting stoma merely decreases the severity of the leak or actually prevents it [60, 70, 75, 105–111]. A recent Cochrane review reported the decreased incidence of anastomotic leak and the need for urgent return to the OR for leak (RR = 0.33, 95 % CI 0.21–0.53) [110]. The placement of pelvic drains is also under debate and several authors have found a decrease in leak incidence while others studies found it to be an independent risk factor for leak [112–119]. Unfortunately, drain use remains largely dogmatic and is most often performed at the discretion of the surgeon.
Presentation and Management
Anastomotic leak presentation depends largely on what level the leak occurs: intraperitoneal or extraperitoneal. Intraperitoneal leaks often present with generalized peritoneal signs, while extraperitoneal leaks are often insidious in presentation due to lack of an innervated peritoneal surface. Patients may only present with symptoms associated with the location such as urinary dysfunction. Leaks can further be broken down into free and contained leaks. A free leak occurs when fecal contents spread freely throughout the peritoneal cavity. A contained leak occurs when fecal contents leak into the pelvis and become walled off, resulting in a pelvic abscess. Free leaks present with signs of sepsis and diffuse peritonitis or feculent fluid from the incision or drains. Contained leaks can present with sepsis, but symptoms such as chronic pelvic pain or fistula or more indicative of a contained leak. Management of intraperitoneal and extraperitoneal leaks is summarized in Figs. 53.1 and 53.2.
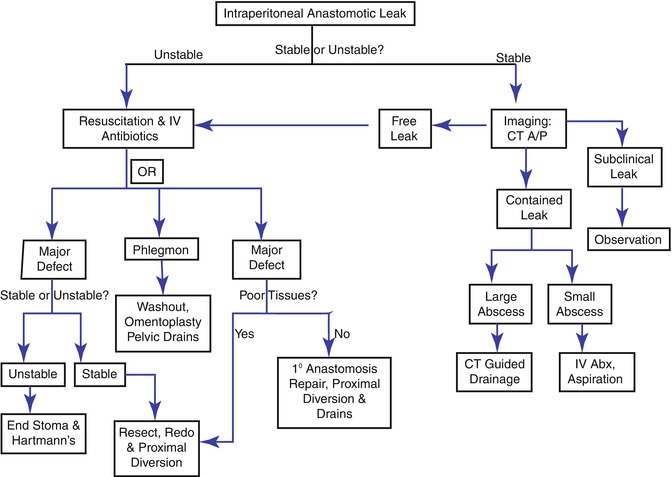
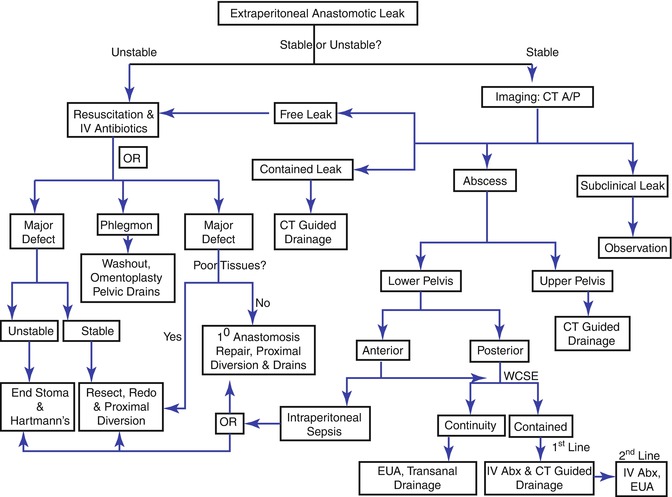

Fig. 53.1
Intraperitoneal leak management algorithm

Fig. 53.2
Extraperitoneal leak management algorithm
Hemodynamically unstable patients need immediate fluid resuscitation, IV antibiotics and operative intervention to assess the size, site, accessibility, viability of the bowel ends, and fecal load of the proximal colon. If there is a major defect in the anastomosis (Fig. 53.3), it can be resected and re-done with proximal diversion, drain placement and on-table colonic lavage. If the anastomosis is high or mid-rectal, there are several options. Minor defects can occasionally undergo repair with proximal diversion and drain placement if there is no fecal load in the proximal colon, and the patient is stable with a reasonable nutrition status. However, this is likely only possible when the leak is identified very early and there is a paucity of inflammation in the abdomen and the bowel wall remains supple. Finally if the patient is too unstable and damage control surgery is planned, then a takedown of the anastomosis with creation of a colostomy and Hartman’s stump is the quickest and safest option. All repairs or re-do attempts should have thorough washout of the abdominal cavity and omental flap over the repair to prevent fistula formation. If a defect cannot be defined and phlegmon is present, minimal pelvic dissection is undertaken to prevent the liberation of sepsis, minimize the injury to ureters or the iliac vessels or to the bowel. The pelvis should be washed out, an omentoplasty performed and pelvic drains placed.
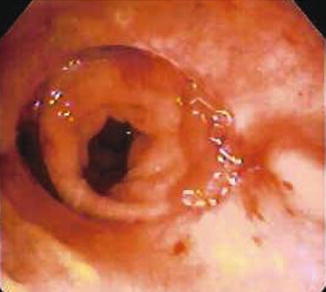

Fig. 53.3
Anastomotic disruption in a diverted patient viewed endoscopically
Hemodynamically stable patients need a triple phase CT to delineate location and size of the leak or abscess. Intraperitoneal abscess or contained leaks are eligible for non-operative management. This consists of drainage, antibiotics, bowel rest and TPN or a low residual diet. For upper pelvis abscess or contained leak in colorectal anastomosis, CT-guided percutaneous drainage can be undertaken with transabdominal route preferred over the transgluteal. Lower pelvic abscess are managed to according to their location: anterior or posterior. Anterior abscess with intraperitoneal sepsis require operative intervention. Posterior abscess require a water-soluble contrast enema (WCSE) to determine if is in continuity or contained. Abscess that is not in continuity should undergo EUA and transanal drainage. For those in continuity, IV antibiotics and CT-guided drainage is first-line therapy. Anterior abscess without intraperitoneal sepsis are managed in a similar fashion to posterior abscess. Colo-anal contained anastomosis leaks benefit from early EUA with frequent reassessment and trans-anastomotic drainage. While endoluminal stents have been described, their use remains largely anecdotal and recommendations await further experience. Long-term sequelae of anastomotic leaks include fistula formation, stricture, chronic presacral cavity, pain and the need for a permanent stoma [51, 120].
Infection and Wound Complications
Incidence
Surgical Site Infection (SSI) is one of the most common nosocomial infections among surgical patients, occurring in approximately 2 % of surgical procedures and accounting for 20 % of health care-associated infections [121]. Colorectal surgery is associated with an even higher rate of SSI (up to 30 %), secondary to high bacterial load in the distal gastrointestinal tract [122, 123]. Within colorectal surgery, procedures on the rectum carry a higher risk of SSI compared to other sites within in the colon. A prospective study of patients undergoing elective colorectal surgery stratified the data from colon and rectal cases separately. SSI was documented in 18 % in rectal cases compared to 9.4 % in colon cases. Italian investigators further stratified the location of surgery to right and left colon and rectum as well as site of infection (incisional or deep space). Again, rectal surgery was associated with a higher rate of SSI compared to the right colon (17.6 % vs. 8 %), while left colon surgery had a similar rate of SSI (18.4 %, p = 0.022). Rectal surgery and left-side colon surgery also had a higher rate of deep space and organ infections compared to right-side colon surgery (p = 0.029) [122, 124]. Risk factors for all surgery and specific to rectal surgery are summarized in Table 53.2 [103, 104, 122, 124–129].
Table 53.2
Risk factors and prevention for SSI
General risk factors | General prevention measures |
Malnutrition | Tobacco cessation |
Diabetes mellitus | Alcohol cessation |
Immunosuppression | Improved nutrition status |
Age >60 | Prophylactic antibiotics with appropriate gram-negative and anaerobic coverage with appropriate timing of dosing and discontinuation within 24 h of surgery |
ASA >2 | Hair removal using clippers |
Increased preoperative hospital stay | Meticulous technique and dissection |
Extensive surgery | Avoidance of excessive intraoperative blood loss |
Normothermia maintenance | |
Independent risk factors in rectal surgery | Specific prevention measures |
Multiple co-morbidities | Combined non-absorbable oral and parenteral antibiotics |
Preoperative use of steroids | Postoperative oxygen therapy |
Ostomy creation | Silver-impregnated dressings |
Preoperative radiation | Intra-operative antibiotic peritoneal lavage |
Antibiotic impregnated beads for APR sacral cavity |
Prevention
Prevention involves modification of general risk factors as well as the use of adjuncts when appropriate, also summarized in Table 53.2 [103, 104, 122, 124–129]. Mechanical bowel preparation is believed to prevent SSI by reducing the fecal content and thus bacterial load, but multiple studies demonstrate no benefit in prevention of SSI. Furthermore, a recent meta-analysis suggests that anastomotic leak rate might actually be increased. In this study the use of rectal enemas in both colonic and rectal surgery was also examined. An overall leak rate of 4.4 % in the enema group was comparable to 3.4 % in the no enema group (OR = 1.32, 0.74–2.36). Specifically in rectal surgery, leaks were present 7.4 % in the enema group and 7.9 % in the no enema group (OR = 0.93, 0.34–2.52). SSI was present 9.9 % in the enema group and 8.0 % in the no enema group (OR = 1.26, 0.85–1.88) [130]. Oral antibiotics have also been examined as a preventive measure. While studies show there is a reduction with use of non-absorbable oral and parenteral antibiotics in colorectal surgery, there is also a higher rate of nausea and vomiting in these patients which predisposes them to aspiration so use can be considered in carefully selected patients [131].
Management
SSI typically present on the fifth to seventh postoperative day unless Clostridium perfringens or beta-hemolytic Streptococcus is the etiological agent, in which case infection manifests as early as postoperative day 1 or 2. Surgical wounds appearing infected require the standard wound treatment of drainage and debridement. Antibiotic should be given only if cellulitis is present, or in patients with underlying immunosuppression. Deep wound infections of the fascia or muscle require return to the operating room for exam under anesthesia, debridement and washout with packing and closure by secondary intention. Larger wounds may benefit from use of vacuum-assisted device, which allow for easier wound care and faster closure of the wound. Patients with suspected intra-abdominal abscess should undergo CT scan with intravenous, oral and rectal contrast. The findings of a rim-enhancing fluid collection and surrounding inflammatory stranding are diagnostic. Treatment is drainage of abscess with most amenable to percutaneous catheter drainage. Success rates range from 65 to 90 % and depend on size, complexity, etiology and microbial flora [65, 132–134].
Wound Issues in APR
Wound issues in APR are a well-known complication and can range from minor wound separation to a chronically infected cavity, fistula or sinus. The rates of wound complications range from 11 to 50 % [135]. Risk factors vary and the importance of one over the other has not been determined, although some factors such as preoperative radiation are consistently a risk factor for delayed wound healing, with rates of 2–3 times to that of no radiation treatment. Other factors such as diabetes, smoking, gender, steroid use and malnutrition have differed from study to study [135–143]. Prevention of wound complications is aimed at modifiable risk factors such as nutritional status and tobacco cessation, prevention of intra-operative fecal or purulent contamination and excessive intraoperative bleeding. The use of pelvic drains is also felt to be beneficial by eliminating the dead space; however, benefit did not extend beyond 1 month. The drain(s) should brought out through a separate incision, because of higher rates of non-healing when exiting through the incision [144, 145]. With preoperative radiation or extensive resection, the transposition of healthy tissue such as omentum or gracilis or rectus abdominus muscle was reported in some studies to improve wound healing, but a systematic review did not show benefit with use of omentoplasty [146–149]. In recalcitrant chronic wounds, a search for other etiology such as fistula or recurrence of malignancy should be initiated. Imaging is utilized to determine the presence of a chronic sinus or fistula. Treatment should be based on standard principles of fistula management, depending on the source, output and clinical condition of the patient. Chronic draining sinus is managed in a similar fashion to pilonidal disease with curettage and excision of chronic granulation tissue, hair follicles and skin bridges. Large wounds may require the use of vacuum-assisted closure or myocutaneous flaps such as the gracilis muscle for closure and healing [135, 150–153].
Functional Issues
With improvement in surgical technique and increasing use of multi-modality treatment, overall survival from rectal cancer has improved drastically. This produces a double-edge sword in which patients have longer life expectancy but have the potential for with functional disturbances such as incontinence and fecal, urinary and sexual dysfunction. Table 53.3 summarizes the risks associated with functional issues.
Table 53.3
Risk factors with functional issues
Fecal incontinence |
Preoperative radiation therapy |
Anastomotic height |
Tumor height |
Intra-operative blood loss >1400 mL |
Pudendal or levator ani nerve damage |
Urinary dysfunction |
Low rectal cancer, <5 cm from anal verge |
Lymph node involvement |
History of urinary dysfunction |
Anastomotic leak |
Suture entrapment |
Preoperative radiation therapy |
Electrocautery injury |
Sexual dysfunction |
Patient age |
Preoperative libido dysfunction |
Preoperative radiation therapy |
Low rectal tumor requiring APR |
Low anterior resection syndrome |
Anastomotic leak |
Preoperative radiation therapy |
Poor trans-anal stapling technique |
Excessive circumferential margins in TME |
Fecal Incontinence
Normal continence is a complex process requiring integration between sphincters, pelvic floor muscles, stool volume and consistency, rectal compliance and intact peripheral and central nervous systems. The inferior hypogastric plexus supplies autonomic function of the rectum, organs of the genitourinary tract, bladder and urethra. This coarse, flat meshwork of nerves contains sympathetic and parasympathetic nerve fibers. Parasympathetic fibers are supplied by pelvic splanchnic nerves (nervi erigentes) originating from sacral nerves S2 to S4. The hypogastric nerves supply the sympathetic fibers of the plexus [154–157]. During dissection of lateral planes in deep portions of the pelvis, both inferior hypogastric plexus and pelvic splanchnic nerves are at risk for injury. To preserve autonomic nerve function, autonomic nerve preserving TME (ANP-TME) was described in the mid-1970s by Tsuchiya and colleagues [158, 159]. However, the prevalence of fecal incontinence still remains high, with reports of up to 40 %, and preoperative radiation therapy (PRT) increasing the rate up to 60 % [160–162]. There is no general consensus for the most influential independent risk factors, however, PRT, anastomotic and tumor height, excessive intra-operative blood loss and damage to pudendal or levator ani nerves are all implicated [154, 161–171].
Work up of fecal incontinence begins with a thorough history including preoperative level of fecal and urinary continence. Physical exam involves visual inspection of the perianal area and digital rectal exam to assess anal sphincter tone. Diagnostic studies include endoscopic anal ultrasound, anorectal manometry, pudendal nerve terminal motor latency, electromyography and defecography [172]. Treatment depends on etiology, but should begin with conservative medical management measures first. Bulking and constipating agents such as fiber, laxatives and loperimide are all recommended but there is actual little evidence of efficacy in functional incontinence [173]. Retrograde transanal irrigation is reported as a means to relieve incontinence difficulties. This is achieved by instilling lukewarm water in the anal canal and washing out the fecal contents [173]. In a small series of rectal cancer patients, this was effective in 79 % of patients; however, the technique is time and resource consuming with fecal soiling still occurring after correct performance of technique. [174–177] For incontinence from sphincter or neuronal injury, sacral nerve simulation provides marked improvement in function in up to 80 % patients. Several studies evaluated the use of SNS in rectal cancer patients and the results have been positive [178–182].
Urinary Dysfunction
The incidence of urinary dysfunction is also high and ranges from 30 to 70 %. It is the most common early post-operative complication in APR [183–186]. Urinary dysfunction includes retention, stress incontinence and urgency, with retention as one of the more common forms of dysfunction. Bladder contractility is under control of parasympathetic fibers via pelvic branches of inferior hypogastric plexus. Damage to these nerves results in denervation of the detrusor muscle, causing a partial paralysis. This manifests as a hypo- or acontractile bladder with decreased sensation resulting in retention [187, 188]. Damage to the hypogastric nerves results in stress incontinence and urgency in female patients. Predictive factors for bladder dysfunction include a low rectal cancer (<5 cm from the anal verge), lymph node involvement and a history of urinary dysfunction prior to surgery [154, 189, 190]. Extensive work-up is not required and treatment consists of bladder decompression for 5–7 days post-operatively. While most patients experience resolution of symptoms within 3 months post-operatively, persistent symptoms benefit from urologic consultation for further work-up [191]. Continued difficulties 6 months postoperatively are likely to have permanent problems requiring management via intermittent self-catheterization [191, 192].
Sexual Dysfunction
The incidence of sexual dysfunction in males undergoing APR is reported as 15–50 %, while those undergoing anterior resection and TME report impotence ~20–46 % and ejaculation failure 20–60 % [184, 193]. In women, underreporting obscures incidence, but may be 10–20 % [194, 195]. Postoperative dysfunction is usually the result of nerve damage, but can be dependent on multiple factors to include: patient age, preoperative libido, preoperative radiation therapy, lack of a standard definition for sexual dysfunction, and social and cultural barriers to discussing sexual complaints. Overall, the type of dysfunction is related to pattern of nerve injury. In males, damage to superior hypogastric plexus or hypogastric nerve results in ejaculatory difficulties such as retrograde ejaculation. Damage to inferior hypogastric plexus, pelvic splanchnic or cavernous nerves result in erectile dysfunction. In females, damage to parasympathetic and sympathetic nerve fibers results inability to produce vaginal and vulvar lubrication. Dyspareunia occurs with injury to the cavernous fibers and inferior hypogastric resulting in denervation of the vaginal wall, decreased lubrication and loss of suppleness [155, 184, 196].
Management is tailored to the patient and involves a frank discussion preoperatively and post-operatively to effectively manage patient expectations. For males, treatment of erectile dysfunction should be multi-modal with use of medications such as phosphodiesterase-5 inhibitors (i.e., Viagra) and psychotherapy to enhance pharmacological treatment [193, 197]. Penile prosthesis are also effective, but extremely invasive and irreversible, and normally are a consideration as a last resort after other measures have failed [193]. In females, treatment is based primarily on psychotherapy particularly for libido disorders. The use of systemic estrogen therapy is reported but only for short-term courses given increased risk of thromboembolic events and endometrial cancer [198]. Topical estrogen therapy is given to improve vaginal lubrication and vulvar atrophy [68, 199]. Phosphodiesterase inhibitors are an option for refractory vaginal dryness and vulvar atrophy. Improved clitoral sensitivity is also reported [200]. Dyspareunia or vaginumus is managed with pelvic floor rehabilitation using Kegel exercises and biofeedback with good results. There are no standard surgical treatments for these conditions although vestibulectomy or perineoplasty has been reported [193].
Low Anterior Resection Syndrome
Low anterior resection syndrome (ARS) is severe bowel dysfunction resulting in incontinence of flatus, feces, urgency and frequency that occurs after low anterior resection. Reported incidence is 10–20 % and apparently related to location of anastomosis in proximity to the anal verge. Anastomoses within 3 cm of the anal verge have increased severity of ARS compared to an anastomosis within 6 cm of the anal verge [165, 201–204]. Ultra-LAR has higher incidence of ARS with one case series reporting 30 % [205]. ARS is thought to occur by one or more of several pathophysiologic mechanisms: rectal reservoir dysfunction, colonic dysmotility or anal sphincter damage [205–207]. Prevention is somewhat achieved by limiting the amount of radiation delivered to the sphincter when possible. 3DXRT with full or partial sphincter blocking reduces the amount of radiation delivered to the sphincter complex [208]. Similarly, technical considerations with avoidance of undue pelvic trauma to surrounding tissues and the sphincter mechanism.
Signs and symptoms of ARS include a mix of high bowel frequency per day with liquid stools, multiple evacuations within a limited amount of time period, urgency and fecal incontinence. Most patients undergoing LAR initially experience this constellation of symptoms and most recover within 6 months of surgery. Work-up begins in those with continued symptoms several months postoperatively [205]. Diagnosis includes documentation of symptoms, number and type of bowel movements per day and objective testing utilized in a standard fecal incontinence work-up. Findings suggestive of ARS are low volume in the neorectum, low evacuation, a wide anorectal posterior angle greater than 110° along with a barium shadow in the anal canal at rest.
Treatment is multi-modal with medical therapy, rehabilitation and surgery, reflecting the multifactorial pathophysiology associated with this syndrome. There is no gold standard algorithm; however, the general consensus is that conservative management should be attempted first with surgical treatment being reserved for a last line therapy [205]. Treatment begins with medical therapy including bulking agents, high fiber diet, valproate sodium, diazepam, topical phenylephrine, amitriptyline and loperimide [209]. Loperamide is usually first line medical therapy for anti-motility effect and increase in anal sphincter tone [210]. Rehabilitative therapy involves biofeedback with improved success when combined with medical therapy. High-risk patients that classically fail biofeedback include history of XRT, previous anal or pelvic surgery or pelvic organ prolapse. Sacral neuromodulation is available, however more randomized control trials and long-term follow-up are needed [211, 212]. In intractable ARS, surgical management should be sought. Procedures such as sphincteroplasty or sphincteric substitution with gracilis or gluteus transposition or artificial sphincter have been described, though many patients ultimately require a definitive stoma [205].
Late Postoperative Complications
Stricture
Anastomotic strictures are well known to colorectal surgeons, but poorly defined in the literature [52, 213]. One definition is a “chronic narrowing or obstruction to the flow of intestinal contents resulting in clinical signs and symptoms of complete or partial bowel obstruction” [214]. Some define stricture by symptoms such as inability to evacuate stool, while others use the inability to pass a rigid proctoscope or index finger on DRE [215–217]. Stricture is found on screening endoscopy or by patient presentation. There are reports of up to 20 % incidence, but the actual incidence is difficult to determine because of reports of spontaneous resolving “silent strictures” [52, 213]. Strictures may be the result of benign disease, malignancy, radiotherapy, IUDs or iatrogenic with colorectal anastomosis as the most common cause [214, 218]. Risk factors associated with stricture formation are anastomotic leak or ischemia, PRT to the area, stapled anastomosis and lower rectal lesions [52, 216, 217]. A smaller series also reported mucin-producing tumors as a risk factor [217]. A Cochrane review analyzed nine randomized control trials for complications related to stapled versus hand-sewn anastomosis but did not find any differences in evaluated metrics, however stricture was more common in stapled anastomosis with a risk difference of 4.6 % (95 % CI 1.2–8.1 %) and number needed to treat of 17 (95 % CI 12–31) [82]. Treatment of benign strictures is managed by non-operative means with stool-bulking agents to gradually dilate the anastomosis. Other options include use of DRE and Hegar dilators for distal strictures [52]. The use of endoscopic Savary dilation with bougies of increasing diameter has been reported with good success in the literature, although patients who required more than three dilations were not able to achieve normal defecation [219]. Another option is use of pneumatic balloon dilation in symptomatic patients [220]. Predictors of poor response to conservative management include previous radiation therapy, local recurrence of malignancy and prior large anastomotic dehiscence [221].
For those that fail first line treatment, both expanding metallic stents (SEMS) and endoscopic trans-anal resection of strictures (ETARS) have been reported with good results. Complications include bleeding requiring reoperation, asymptomatic anastomotic perforation and technical failure in acutely angled strictures [222]. Other options include biodegradable stents, which have similar efficacy to SEMS, electroincision to produce radial incisions in the scar with balloon dilation, circular/linear stapler resection of the stricture and dilation with concomitant corticosteroid injection [223–225].
Chronic Perineal Pain
Perineal pain is common after anorectal surgery, but is also prevalent following Ultra-LAR for malignancy. Pain is thought to be the result of pelvic floor muscle spasms or levator ani syndrome. The pain usually resolves 2–3 week, but pain continuing beyond a month is considered chronic and an etiology should be sought, as pain may be the result of anastomotic leak or recurrent or residual disease. Work-up to determine etiology, starts with exam under anesthesia. Adjunct diagnostic tools involve imaging such as CT scan or EUS.
Treatment begins with a work-up to exclude local complications or recurrent disease, with directed therapy accordingly. Outside of this, conservative management beginning with warm Sitz Baths and non-steroidal inflammatory medication is warranted. Pelvic floor muscle spasms can benefit from the addition of anti-spasmodics such as diazepam or cyclobenzaprine [135]. Electrogalvanic muscle stimulation is utilized in severe spasms with reported success [226]. Patients with severe spasm also require such adjuncts as Botulinum toxin [227]. For patients that fail all other treatment modalities, APR may be considered. Despite this radical treatment, this procedure has been reported to provide good pain relief to unfortunate patients that are refractory to other measures [135].
Perineal Hernia
Perineal hernia is a rare complication of pelvic surgeries, specifically APR, pelvic exenterations and cystourethrectomy. The incidence is estimated to be 0.2–0.62 %, though historically rates are cited as high as 7 % [228, 229]. Associated risk factors include tobacco use, chemoradiation, malnutrition, wound infection and chronic wound [228–230]. Patients report a “vague dragging sensation” and discomfort upon standing. More rarely, they present with bowel obstruction, urinary symptoms, perineal wound breakdown or pain [226, 230, 231]. When found, this hernia is repaired from either the perineum or the abdomen or a combined approach. Typically the abdominal approach allows superior visualization, avoidance of iatrogenic bowel or vascular injury and ease of mesh placement; however some groups have reported success with the perineal approach [228, 230]. Repair involves reduction, excision of the sac and closure of defect. Defect closure is accomplished with either mesh or autologous tissue, such as gracilis flaps or omentum [135, 226, 228].
53.4 Summary and Conclusion
In summary, while the development of multi-modal therapy for rectal cancer has led to better outcomes and survival, several complications and functional issues can occur. The colorectal surgeon must be well versed in the presentation, work-up and diagnosis of these to allow patients to have a better quality of life after their treatment of rectal cancer.
Key Points
The most common location of bleeding is the presacral venous plexus. Control is difficult to achieve with electrocautery, suture ligation or clipping. Pelvic packing, metallic or titanium thumbtacks are more effective. Other methods include bone wax, endoscopic helical tackers, hemostatic sponges or local agents, tamponading saline bags or indirect coagulation with muscle fragmentation. Anastomotic bleeding is more commonly encountered and should be treated initially with conservative management, followed by endoscopic management.
Genitourinary complications occur infrequently, but are catastrophic if missed. The ureters are the most frequently damaged because of their anatomical location. Ureteral stents do not prevent injury but they do allow for early identification of injuries. Intraoperative injury requires immediate repair. The principles of repair are the use of absorbable sutures to prevent stone formation, a tension-free repair over a stent and placement of closed-suction drain in proximity of repair. The type of repair depends on the location of injury.
In transanal approaches such TAMIS and TEMS, perforation into the peritoneum can occur. This may be successfully repaired transanally, although formal laparotomy may be required. Diversion is typically only required with delayed presentation.
Anastomotic leak is a devastating complication. Principles of prevention include mitigation of modifiable patient risk and intraoperative factors, along with the use of adjuncts. Intra-operative adjuncts include the use of buttressing materials, air leak testing the anastomosis and a restrictive resuscitation strategy. In the post-operative period, supplemental oxygen therapy, the use of protective diverting stomas and pelvic drains are also reported.
Management of an anastomotic leak depends on whether it is contained or free. Contained leaks in hemodynamically stable patients should undergo CT scan imaging and can be managed with conservatively with percutaneous drainage, antibiotics and bowel rest. Hemodynamically, unstable patients and free leaks should undergo resuscitation with intravenous fluids, broad spectrum IV antibiotics and return to the operating room for operative intervention to assess the size, site, accessibility, viability of the bowel ends, and fecal load of the proximal colon. Anastomotic location and the status of the patient determine the procedure to be performed, but generally involve diversion or repair (in select cases).
Surgical site infections are common following colorectal surgery with proctectomies having a higher rate of SSI. Oral and systemic antibiotics have shown to reduce the rate of SSI in rectal cases, but the use of mechanical bowel preparation has not. Intravenous antibiotics should have appropriate gram negative and anaerobic coverage and be dosed appropriately. Aggressive wound infection management should be undertaken to prevent further spread.
Functional issues such as low anterior resection syndrome, urinary dysfunction and sexual dysfunction are relatively common occurrences after rectal cancer surgery. Patients should be counseled preoperatively regarding these matters. Most will resolve several months after surgery and can be managed conservatively, but appropriate work-up is required to determine cause and course of management to be taken.
Fecal incontinence following proctectomy is multi-factorial, but can usually be managed with medical management with bulking or constipating medications. Evaluation involves physical examination and objective testing such as endoscopic anal ultrasound, anorectal manometry, pudendal nerve terminal motor latency, electromyography and defecography. Sacral nerve modulation has shown promise. Surgery is reserved for failures and may require permanent diversion.
Anastomotic stricture following restorative procedures may occur, though the true incidence is difficult to determine. Strictures may be found on screening endoscopy or by patient symptoms. Causes include recurrent, malignancy, radiotherapy, or ischemia at the anastomosis. Treatment of benign strictures is managed by non-operative means with stool-bulking agents to gradually dilate the anastomosis or endoscopic serial dilation. Other options include biodegradable stents, which have similar efficacy to SEMS, electroincision to produce radial incisions in the scar with balloon dilation, circular/linear stapler resection of the stricture and dilation with concomitant corticosteroid injection. Recurrent disease should have a complete oncological work-up and then resected accordingly.
Perineal pain is common after surgery, especially ultra-LAR. Pain is thought to be the result of pelvic floor muscle spasms or levator ani syndrome. The pain usually resolves in 2–3 week, but pain continuing beyond a month is considered chronic and an etiology should be sought. Work-up to determine etiology includes an exam under anesthesia, along with adjunct diagnostic tools such as CT scan or EUS. Treatment includes conservative management with warm Sitz Baths, non-steroidal inflammatory medication, bulking agents, and anti-spasmodics for pelvic floor muscle spasms. Diversion may be required in rare cases.
References
1.
Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








