Fig. 3.1
Ureteral aspirate from a patient with a low grade papillary urothelial carcinoma of the ureter. Tumor cells have slightly enlarged and crowded nuclei. (a) Thinprep, Papanicolaou stain ×400. (b) Cell block, H&E stain ×400
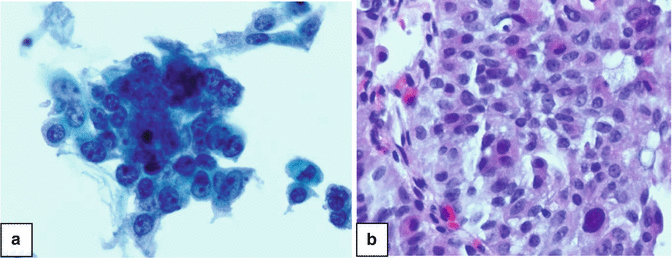
Fig. 3.2
High grade urothelial carcinoma of ureter. Note enlarged nuclei with coarse chromatin and nucleoli. (a) Thinprep, Papanicolau stain ×600. (b) Cell block, H&E stain ×600
Key Features of Low Grade Urothelial Carcinoma
Umbrella cells variable
Haphazard growth pattern
Large cells
High N/C ratio
Irregular nuclear membranes
Small nucleoli
Granular chromatin
Mitoses infrequent
Key Features of High Grade Urothelial Carcinoma
Absent umbrella cells
Haphazard growth pattern
Large cells
High N/C ratio
Irregular nuclear membranes
Prominent nucleoli
Coarse chromatin
Mitoses frequent
Cytoplasmic differentiation (glandular/squamous)
Caution in the interpretation of cytologic samples is necessary because specimens from the upper urinary tract contain large number of superficial cells and may show some multinucleation or atypia not seen in catheterized bladder urine cells and can be misinterpreted as malignant. Adherence to clear cut nuclear criteria of malignancy will prevent the misdiagnosis.
Post laser ureteral washings show an artifact of cellular spindling (Fig. 3.3), which occur in single cells or loose clusters and have elongated nuclei with dense chromatin. The changes are due to an epithelial response to heat.
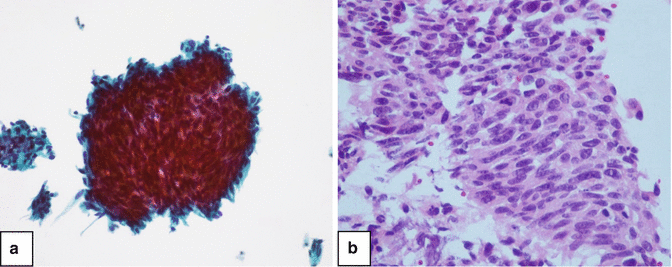

Fig. 3.3
Low grade urothelial carcinoma of the ureter after laser treatment. (a) Thinprep, Papanicolau stain ×200. (b) Cell block, H&E stain ×400
Metastatic carcinoma to the upper urinary tract needs to be considered in the differential diagnoses, though rare, such as renal cell carcinoma (Fig. 3.4).
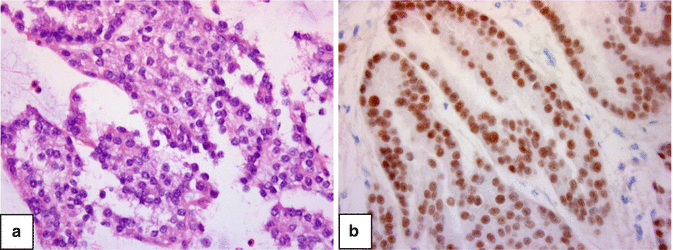

Fig. 3.4
Papillary renal cell carcinoma metastastic to the ureter. (a) Cell block H&E. ×400. (b) Pax 8 immunostain ×400 (a marker for RCC)
3.3.2 Histologic Specimens
Multiple grading schemes have been developed and employed for urinary tumors throughout the years such as the WHO 1973 classification [11, 12]. This classification includes urothelial papilloma and three grades of papillary carcinoma (grade 1, grade 2, and grade 3). This classification allows intermediate grades such as grade 1–2 and grade 2–3 to reflect tumor heterogeneity. Urothelial carcinoma grade correlates with the probability of recurrence, invasion, and metastasis, in addition to survival [13–16]. Presently we follow an adapted version of the 2004 World Health Organization (WHO) classification for UTUC [17]. This classification includes papillary urothelial neoplasm of low malignant potential (PUNLMP), low grade carcinoma and high grade carcinoma. The relationship between WHO 1973 and 2004 is shown in the Table 3.1.
Table 3.1
Relationship between tumor grading systems
WHO 1973 | WHO 2004 |
|---|---|
1 | PUNLMP |
1–2 or 2 | Low grade carcinoma |
2–3 or 3 | High grade carcinoma |
At Jefferson, samples from upper urinary tract are interpreted by pathologists with experience in grading UTUC (Figs. 3.1, 3.2, 3.3 and 3.4).
Our ability to diagnose and grade UTUC has markedly improved since adopting the described sample processing in the cytology laboratory, from 40 to 90 % combining cytospin and cell block specimens [8].
There has been concern regarding ureteroscopic biopsies not reflecting the actual tumor grade. In one of our studies comparing ureteroscopic biopsies and cytologic specimens with open surgical specimens of urothelial tumors, accurate information regarding grade and stage was obtained. Grading of ureteroscopic specimens was possible in 82.4 % and accurately predicted tumor grade and stage in the surgical specimens [18]. Williams et al. [19] have also demonstrated concordance between endoscopic biopsies and final pathologic specimens.
3.4 Adjunct Testing
Grading UTUC may be challenging and inter-observer agreement among general pathologists is poor. Cytology alone was found to be poorly sensitive (40 %) but highly specific (79 %) for the detection of UTUC [1–8].
Fluorescence in situ hybridization (FISH) was found to increase the sensitivity of cytology and decrease specificity [20] but it also resulted in many false-positive cases. Johannes et al. [21] reported a limited value of FISH for upper tract tumor surveillance.
Some studies have investigated the prognostic impact of various biologic markers that are related to cellular processes such as cell adhesion, cell differentiation, angiogenesis, cell proliferation, epithelial mesenchymal transition, mitosis, apoptosis and vascular invasion [22, 23].
p53 is an important factor in cell cycle regulation and was found to be a predictor of UTUC patient survival in univariable analyses, it did not emerge as an independent prognostic factor after adjustment for other clinical and pathologic characteristics [24–29].
The overexpression of Ki-67, a protein involved in cell proliferation, was found to be associated with advanced tumor stage and higher grade and to be an independent predictor of survival [30].
Epidermal growth factor receptor (EGFR) is strictly related to cell growth, proliferation, and differentiation. The overexpression of EGFR was found to be associated with advanced UTUC and metaplastic differentiation but not with cancer-specific survival in multivariable analysis [31].
Several apoptosis-related markers have also been investigated in patients with UTUC. The overexpression of survivin and Bcl-2, although associated with higher tumor grade and stage, was not associated with patient survival, according to a retrospective study by Nakanishi et al. [32].
Angiogenesis is essential for human tumor growth. Increased levels of hypoxia-inducible factor 1a were found to be associated with both recurrence-free survival and cancer-specific survival by two single institutional studies, even after adjustment for several clinicopathologic variables [33, 34]. Similarly, an increased expression of metalloproteinases was shown to correlate with cancer aggressiveness and to be an independent predictor of prognosis in two studies [35, 36].
The prognostic value of molecules involved in cell adhesion was also evaluated by several investigators. A lower expression of E-cadherin was shown to be associated with higher tumor stage and grade [37]. E-cadherin was confirmed as an independent factor when predicting recurrence-free survival and cancer-specific survival [35, 38].
Microsatellite instability (MSI) is an independent molecular maker used for tumor prognosis [30]. In addition, MSI can help detect germ-line mutations, allowing for the detection of possible hereditary cancers.
Solomides et al. [39] investigated the use of a mitotic specific marker phospho-histone H3 –PHH3 as an adjunct to H&E stain for grading UTUC in cell blocks. The inter observer agreement in tumor grading improved dramatically by adding the PHH3 stain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






