Fig. 28.1
Pathogenetic mechanisms of inflammatory bowel diseases
Epidemiology
Although IBD can occur at any age, up to 25 % of patients develop symptoms during childhood and adolescence [1, 2]. Some epidemiological studies from USA and Europe have shown a steady inrise in the overall mean annual incidence of pediatric IBD around the world, that seems to be primarily due to an increase in the incidence of CD [1, 2, 6]. CD is unequally distributed all over the world, with highest rates occurring in Western and Northern countries, and with a decreasing gradient from North to South and from West to East [7–11]. The worldwide highest prevalence of pediatric IBD is reported from the Canadian Ontario region, with approximately 56 IBD patients for 100,000 inhabitants [8]. Epidemiological observations from this area, using health administrative data, have indicated an increasing incidence of pediatric CD from 9.5 in 1994 to 11.4 in 2005 per 100,000, with unchanged incidence rates for pediatric ulcerative colitis (UC; 4.1–4.2) [9]. In Europe, a recent study based on the registry of chronic inflammatory intestine diseases in North-West pf France (EPIMAD) indicated a mean annual incidence rate of 2.6 for pediatric CD [11]. The largest population-based study of incidence of pediatric IBD in the USA was reported from Wisconsin, estimating an yearly incidence of IBD of 7.05 per 100,000, the incidence of CD being 4.56, that is, more than twice the rate of UC (2.14) and a prevalence of 48–71 per 100,000 [12]. A recent study estimating the prevalence of IBD in the USA using a large, multistate sample, reported a prevalence of CD in children of 43/100,000 [13]. Factors contributing to the evident increase in the global incidence could be a greater case ascertainment, the widening case definition, earlier onset in predisposed individuals, and greater access to health care; however, there is a wide agreement that the rising incidence of pediatric IBD is due to a real increase in the number of affected children [14]. It has been postulated that the “Westernization” of different societies accounts for the progressive rise in the incidence of the disease also in previously low-incidence areas, including Japan [15], other Asian countries [16], and some Eastern European countries [17] .
Pediatric IBD present specific phenotypic and demographic differences when compared with adult-onset disease. While CD and UC occur with equal distribution in adults [18], it has been reported that in childhood CD is more frequently diagnosed than UC [19, 20]; moreover, while in adults there is an equal male to female ratio (or a mild female predominance), all pediatric IBD cohort studies or registries indicate a male predominance [20]. In pediatric CD, most patients have an extensive disease, ileocolonic or colonic, and distinction of UC from colonic CD may be not uncommonly challenging [21]. As recently confirmed by EPIMAD registry, children with CD are more likely to have upper gastrointestinal involvement than their adult peers [11]. Moreover, disease severity seems to differentiate pediatric-onset CD: children often present an inflammatory or nonstricturing, nonpenetrating disease, while complicated disease is fairly unusual at presentation. However, even with treatment, many data demonstrate that inflammatory CD progresses to stricturing and penetrating disease in several children [11, 20]. Adult disease begins more often with stricturing or penetrating disease, with a lower trend of disease progression than pediatric-onset disease .
Etiopathogenesis
The most accepted hypothesis for the pathogenesis of CD is that the interaction between luminal contents (i.e., the intestinal microbiota) and the mucosa leads to a dysregulated inflammation in a genetically predisposed host. Several microorganisms have been considered as potential causative agents for CD, including Mycobacterium paratuberculosis, Listeria monocytogenes, novel Burkholderiales, Escherichia coli [22, 23]. Recently, strains of adherent-invasive E. coli (AIEC), capable of adhering to and invading epithelium, and to replicate in macrophages, have been described in adults and children with CD [24, 25]. Nevertheless, there are no strong data to support a role for any of these microorganisms as the causative factor in the etiology of IBD. Some of the most interesting findings in the pathogenesis of IBD come from genetics. The importance of genetics in CD was strongly suggested by family, twin, and phenotype concordance studies. Monozygotic twins exhibit phenotypic concordance in 50–70 % of CD patients, and their relative risk of developing CD is 800-fold greater compared to the general population [26]. Recently, the discovery of several susceptibility genes has further supported the importance of genetic predisposition in CD [27]. After the pivotal study of Hugot et al. in 2001, who discovered the association of variants of the NOD2 gene with ileal CD [28], and the discovery of the correlation between variants of interleukin ( IL)23 receptor gene and both CD and UC in 2006 [29], the number of IBD genetic associations discovered has dramatically increased. More susceptibility loci have been quickly identified, such as autophagy genes, ATG16L1 and IRGM [30]. In the past decade, the implementation of genome-wide association studies (GWAS) has significantly advanced our knowledge on the importance of genetic susceptibility in IBD [31, 32]. To date, the GWAS performed have identified more than 70 risk-conferring loci for CD [31]. GWAS have revealed a substantial overlap in genetic risk loci between CD and UC. However, some genes are quite different for CD or UC, clearly denoting the genetic heterogeneity of the two forms of IBD, each one of them showing distinctive and shared genetic associations. For instance, NOD2, autophagy genes, and ITLN1 are unique for CD [31]. Despite the discovery of a massive number of susceptibility genes for IBD, we are still far from understanding the mechanism by which such genetic variants cause the intestinal inflammation . The challenge for basic IBD researchers is now to identify how genetic abnormalities influence pro-inflammatory pathways, providing information that could directly improve the clinical management. A number of pro-inflammatory pathways have been elucidated, in some cases enabling the development of specific interventions. For instance, NOD2 gene-defected patients have an impaired ability to recognize and process bacterial products, and this may lead to an inappropriately innate immune response. Some CD patients with variants of the autophagy genes ( ATG16L1 and IRGM) have a defective capacity to process cell degradation products, as well as bacteria, and therefore an insufficient ability to eliminate pro-inflammatory factors [33]. One of the most important discoveries in the field of genetics of pediatric IBD is the recent identification of impaired IL10 signaling in some forms of very-early-onset (within the first months of life) CD [34]. The common characteristics of these patients are a very early onset of an aggressive CD colitis, treatment-resistant, with perianal involvement. This form of CD is due to homozygous mutations in either IL10RA or IL10RB, which encode subunits of the IL10 receptor, or for IL10 itself [35]. One could speculate that this form of IBD with a monogenetic inheritance could identify a subset of patients with a “more” Mendelian transmission, opening new horizons of research and also expectations to understand the definite mechanisms underlying these diseases.
Clinical Presentations
CD is characterized by a transmural inflammation that can occur anywhere in the gastrointestinal tract, from mouth to anus. While the terminal ileum is the most common site of CD, about 60 % of children have an extensive ileocolonic involvement and 20–30 % an isolated colonic disease [18]. CD typically presents in any age group with a constellation of abdominal pain, diarrhea, weight loss, and poor appetite; however, short stature and predominant perianal disease are further significant presenting features in pediatric CD. Impairment of linear growth and associated delay in sexual development can occur before the onset of intestinal symptoms and can dominate the clinical presentation [36]. Growth failure is a unique characteristic of pediatric-onset CD: It is defined as linear growth at or < 2 standard deviations (SD) below the mean for age, or decreased growth velocity, and can occur in 15–20 % of children with CD [37]. The onset of growth failure is usually insidious, and any child or adolescent with impairment of the linear growth should have an appropriate initial diagnostic evaluation for IBD [18]. The presence of growth and pubertal delay is a key factor in the management of pediatric IBD . Maintaining adequate nutrition, minimizing inflammation, and maximizing corticosteroid-free treatment remain a crucial part of managing the potential growth stunting effects of active IBD, most specifically of small bowel CD. During the clinical course of CD, growth failure has been reported in up to 40 % of children with CD and a final height below the fifth percentile is reported in 7–30 % of patients with pediatric-onset CD [38, 39]. Growth failure in CD originates from different factors, such as malnutrition with decreased intake, increased gastrointestinal losses, malabsorption, and medication effects. All these components can certainly impact an individual’s nutritional state. In addition, it is now widely agreed that inflammation process per se may directly inhibit linear growth and play a major role in the etiology of growth retardation [40]. Inflammatory mediators such as IL-6 and TNF-α are crucial factors in reducing plasmatic levels of insulin-like growth factor 1 (IGF-1), the peripheral mediator of the growth hormone [41]. In fact, impressive catch-up growth can be observed as soon as remission of intestinal inflammation is achieved. It is essential that height, weight, pubertal staging, and bone age are accurately and regularly measured and recorded in young patients with CD. Nutritional supplementation and need for “catch-up” growth should be an important part of the evaluation of a pediatric CD patient. Delay of pubertal onset is also common in active pediatric CD, and also the duration of puberty can be impaired. Active or relapsing disease during the years following the onset of puberty may slow or even arrest the progression of puberty. Moreover, pubertal delay affects estrogen and androgen levels, which are important for normal bone mineralization, contributing to the development of osteoporosis and osteopenia [42]. Inducing and maintaining disease remission before and during the pubertal years is thus crucial in order to ensure the attainment of final predicted height, avoiding a missed pubertal growth spurt.
Diagnosis
There is no gold standard single test allowing a definite diagnosis of CD . It is the combination of family and personal history, physical examination, and subsequent laboratory, imaging, and endoscopic assessment that leads to the correct diagnosis. Nevertheless, a subset of 10–15 % of children with IBD confined to the colon receive an initial diagnosis of inflammatory bowel disease unclassified (IBD-U), and they will be subcategorized only during follow-up [18]. The first diagnostic issue is whether the presenting symptoms are related to CD. In the presence of classic clinical presentation, differentiating CD from other diseases with initial similar symptoms may be relatively simple [43]. However, in a child with bloody diarrhea and infectious etiologies , like Salmonella, Yersinia, Shigella, E. coli, etc., must be excluded. It is also important to evaluate and rule out vasculitides (such as Henoch–Schönlein purpura, and hemolytic–uremic syndrome) or allergic colitis. An abdominal pain mimicking CD may be determined by other conditions, such as intestinal lymphoma, ovarian cyst, appendicitis , or intussusception . Figure 28.2 shows the suggested diagnostic workup of children or adolescents with suspected IBD .
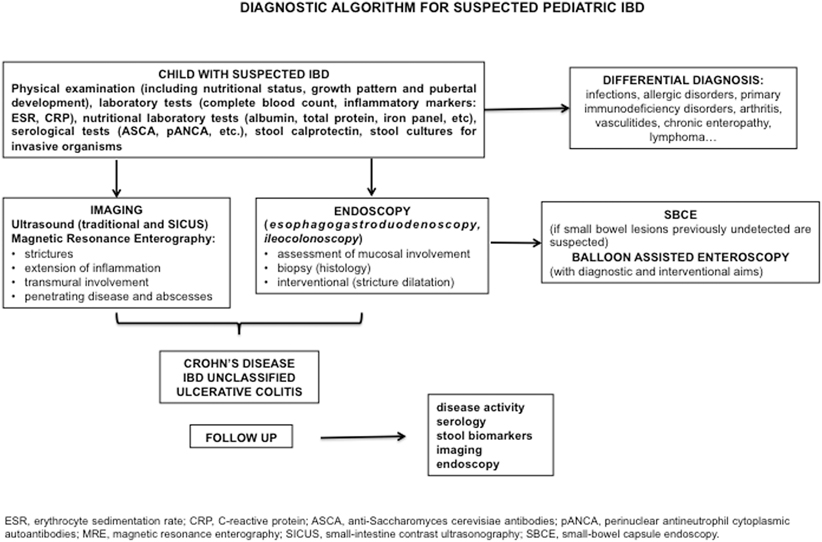

Fig. 28.2
Proposed diagnostic algorithm for pediatric inflammatory bowel diseases
Noninvasive Tests
Serologic Tests
Serologic tests are the first diagnostic tool for suspected CD. They include complete blood count, acute-phase reactants (i.e., C-reactive protein—CRP, erythrocyte sedimentation rate, ferritin, platelet count), and a wide panel of antibodies directed against microbial and self-antigens. CRP levels have been shown to well correlate with clinical, endoscopic, and histologic disease activity, and their stable rise is associated with a higher relapse rate and a better response to biologics [44]. Several antibodies to microbial antigens have been identified in patients with IBD [45]. The most extensively studied and commonly used are anti-Saccharomyces cerevisiae antibodies (ASCA) and perinuclear antineutrophil cytoplasmic autoantibodies (pANCA). ASCAs have been suggested as a marker for CD with a prevalence of 50–60 %, compared with 10–15 % in UC and 0–5 % in healthy controls [46, 47]. Conversely, pANCAs have been proposed as a marker for UC or colonic CD [45]. However, between 30 and 50 % of the IBD patients do not screen positively for either of these markers, so their absence cannot be used to rule out the disease.
The attention of researchers has been recently focused on the possible relationship between serologic markers and distinct subgroups of patients and prognosis of the disease [48]. In CD, positive ASCA titers have been correlated to younger age at onset, ileal disease, aggressive behavior, and increased risk of early surgery [48]. Because of these promising data, other markers useful for prognostic purposes have been identified. Reactivity to Pseudomonas fluorescens–related protein [49] was independently related to stenosing disease and need for surgery, whereas anti-E. coli outer membrane porin C (OmpC) has been associated with penetrating disease [49, 50]. Both the presence and magnitude of the immune response were correlated with more aggressive disease behaviors. Recently, a large, multicenter, pediatric study examined the association of ASCA, anti-I2, anti-OmpC, pANCA, and anti-CBir1 reactivity, with disease behavior. The likelihood of developing disease complications increased in parallel with the reactivity to a greater number of antigens. The odds ratio for the development of penetrating disease was 5.2 and 9.5 for patients with reactivity to 2 and 3 antigens, respectively. Moreover, survival analysis showed that the need for surgery was earlier in patients with a reactivity to at least one microbial antigen as compared to those negative for all markers, suggesting that these markers may predict a more aggressive disease course [51].
Fecal Markers of Inflammation
The neutrophil-derived marker calprotectin is a noninvasive tool for the diagnosis and monitoring of activity of IBD. Calprotectin is a calcium-binding protein that is excreted in the feces and can be readily measured using an enzyme-linked immunosorbent assay (ELISA). The protein is stable in stool specimens for up to a week at room temperature, allowing patients to collect a specimen at home without special precautions. While calprotectin is commonly used in the initial diagnostic approach to suspected IBD, one of the most advantageous uses is its ability to confirm tissue healing and predict disease relapses. A few studies have evaluated this outcome, demonstrating a sensitivity of 89–90 % and specificity of 82–83 % for predicting disease relapse during a 12-month period, with a sensitivity and specificity to predict absence of mucosal healing of 70–100 % and 44–100 %, respectively, depending on the calprotectin concentration threshold used [44]. Other fecal biomarkers, such as lactoferrin, neutrophil-derived S100A12, and high-motility group box 1 (HMGB1), show promise as potential biomarkers for CD, but have been studied far less extensively [50–52] .
Imaging
Most patients with CD have involvement of the small bowel, a region challenging to evaluate. Furthermore, children with CD need frequent evaluations during the course of their disease, so noninvasive imaging test, radiation-free, could be a valuable alternative to endoscopy for the definition of activity and complications of the disease. In the past, small bowel follow through (SBFT) was the “gold standard” for small bowel disease. In the past decade, due to considerable advances in technologies, other modalities, such as magnetic resonance (MR), ultrasound (US), and computed tomography (CT) have been favorably utilized [53]. CT has the major disadvantage of the large radiation exposure, driving interest in alternative imaging techniques to avoid this risk. MR with the administration of oral contrast (MRE) results in luminal distension, facilitating evaluation of bowel wall thickness and regularity; MR enteroclysis (with contrast administered through a nasojejunal tube) seems to have the same sensitivity (88 %) of MRE but a superior patient discomfort [53]. In comparison with endoscopy, MRE has a sensitivity of 84–96 % and a specificity of 92–100 % for the diagnosis of IBD [54]. MRE can also allow a detailed assessment of perianal fistulae, and it should be preferred as imaging tool for perianal disease [55, 56]. The recent European Crohn’s colitis organization (ECCO) and European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines on the management of adult and pediatric CD, respectively, recommend MRE as the imaging tool of choice for the diagnosis and follow-up of CD [57, 43]. Abdominal US is a widely used technique in the evaluation of patients with CD because of its excellent safety profile, low cost, and recent advances in the equipment (Doppler and use of oral contrast) that allow high-resolution images [58]. Recently, a small intestine contrast ultrasonography (SICUS), performed after ingestion of an oral contrast material filling the small bowel lumen with anechoic fluid, has been developed for the study of the small bowel [59]. SICUS provides the opportunity to visualize and assess the entire small bowel by measuring intestinal wall thickness and lumen diameter at different levels [59, 60]. This technique is able to detect intestinal lesions in patients with suspected small bowel diseases with a higher sensitivity (72–100 %) and specificity (97–100 %), compared with SBFT [60] .
Endoscopy
Ileocolonoscopy and Esophagogastroduodenoscopy)
Traditional endoscopy has a pivotal role in the diagnosis of suspected CD. According to the recent ESPGHAN guidelines on the diagnosis of IBD [43], ileocolonoscopy and EGD should be recommended as the initial workup for all children with suspected disease. Multiple biopsies should be obtained from all sections of the examined gastrointestinal tract, even in the absence of macroscopic lesions [43]. Beyond its diagnostic utility, traditional endoscopy is crucial for staging the severity of disease, for the evaluation and treatment of strictures, detection of postoperative recurrence, surveillance for neoplasms, and preoperative assessment. Moreover, endoscopy allows monitoring of response to therapies by evaluating mucosal healing. In the past decade, endoscopic healing has become the most rigorous end point in adult therapeutic trial, and it is being used in pediatric trials too [61, 62]. Tables 28.1 and 28.2 show the typical endoscopic and histologic findings differentiating CD and UC. Peculiarities of endoscopy and histology in pediatric IBD have been defined in an exhaustive fashion in the recently published Porto Criteria of the ESPGHAN [43].
Table 28.1
Endoscopic differentiation between typical CD and UC
CD | UC |
|---|---|
Throughout the entire GI tract | Confined to colon |
Discontinuous lesions | Usually continuous lesions |
Rectal sparing or segmental inflammation | Rectal involvement (in children may be absent) |
Aphthous ulcers (may occur in normal mucosa) | Mucosal granularity/friability |
Linear ulcers common | Erosions/microulcers |
Cobblestoning | Loss of vascular pattern |
Ileocecal valve stenotic and ulcerated | Ileocecal valve patulous and free from ulcerations (possible backwash ileitis) |
Table 28.2
Histologic findings in CD and UC
CD | UC |
|---|---|
Focal crypt distortion | Mucosal surface alteration |
Ulcers and/or aphthoid ulcers | Crypt distortion, atrophy |
Mucin depletion absent or weak | Mucin depletion |
Pseudopyloric metaplasia | Cryptitis and/or crypt abscesses |
Focal cryptitis | |
Focal lymphoplasmacellular infiltration in the lamina propria | Diffuse lymphoplasmacellular infiltration in the lamina propria |
Granulation tissue-like inflammation | Basal plasma cell infiltration |
Epithelioid granulomas |
Small Bowel Capsule Endoscopy
Small bowel capsule endoscopy (SBCE) allows the evaluation of CD small bowel lesions, with no radiation. It is very sensitive for determining mucosal lesions, but it cannot detect extraluminal processes and it does not allow the biopsy of detected findings. In comparison to traditional endoscopy, SBCE can evaluate the entire small bowel, has an easier preparation, and is better tolerated from children. Regardless of its many advantages, SBCE also has some weaknesses: biopsy or intervention is not possible, and there is no way to guide the capsule, so significant lesions may be missed because of a bad orientation of the camera, obscured visualization due to luminal bubbles or debris, or delayed intestinal transit resulting in an inaccurate exam. SBCE is contraindicated in patients with strictures because of the risk of capsule retention. Furthermore, there are no established diagnostic criteria for CD: although most studies have defined the presence of more than three ulcerations in the absence of nonsteroidal anti-inflammatory drug ingestion as a diagnostic criterion, this has not been prospectively validated [63]. Given the concerns about the specificity of SBCE in the diagnosis of IBD, but recognizing its good sensitivity, some experts have suggested that it may be useful mainly for monitoring established CD rather than for initial approach [64].
Balloon Enteroscopy
Double-balloon enteroscopy (DPE) was first introduced in 2001 as a device offering the possibility of complete diagnostic and therapeutic access to the entire small bowel with an endoscope [65]. A single-balloon device has also been developed with a similar intention. The enteroscope can be inserted via the oral or anal route and, using the combination of these approaches, complete examination of the entire small bowel can be achieved in many patients [66]. Several studies support the utility of DBE in established adult and pediatric CD with the potential to affect management in select populations of patients (small bowel disease) [67]. The main limitations of balloon-assisted enteroscopy (BAE) are its invasive nature with the risk of bleeding and perforation (complication rate for diagnostic procedures is around 0.8 % but can be as much as 4 % for therapeutic interventions), prolonged duration, limited evaluation of the entire small bowel in a one-step approach, and requirement for specialized personnel [53].
Therapy
The current goals of treatment of pediatric CD are to induce and maintain a prolonged remission, minimizing drug toxicity. At long term, they should include preventing relapses, optimizing growth and pubertal development, and improving quality of life. Modern expectations of CD therapy call also upon the concept of mucosal healing, and, eventually, of deep remission (i.e., clinical remission, biomarker remission, and mucosal healing) [68]. The introduction of these outcomes may be the best way to alter the natural course of these diseases by preventing disability and bowel damage [69]. This may be of clinical relevance in children with CD, given the long-term consequences of early-onset aggressive disease presentation. Pediatric CD therapy employs many of the same treatment regimens as their adult counterparts. There are only a few well-designed clinical trials performed in children, therefore much of the evidence given is based on adult data. Conventional therapy is based on the escalation of drugs, from those with a better safety profile but a lower efficacy (antibiotics, mesalazine, sulfasalazine) to those with improved efficacy but a greater risk of side effects (steroids, immunomodulators, biologicals, surgery). This “step-up” approach is applied in most cases of pediatric IBD (Fig. 28.3). The advantages of “step-up” management are mainly to reserve more toxic drugs for those patients with a demonstrable “need” for more intensive therapy [70]. However, potential disadvantages include the observation that conventional therapies have not altered the disease course towards disease complications (strictures and fistulae) or the need for surgical procedures. Hence, pediatric gastroenterologists are moving towards an “early aggressive” approach, with the aim of changing the natural history of the disease [70]. So far, there are no definite proper criteria to select which patients will certainly benefit from an early aggressive approach [71]. The knowledge of these genetic, laboratory, or clinical markers will be the challenge in pediatric IBD research.
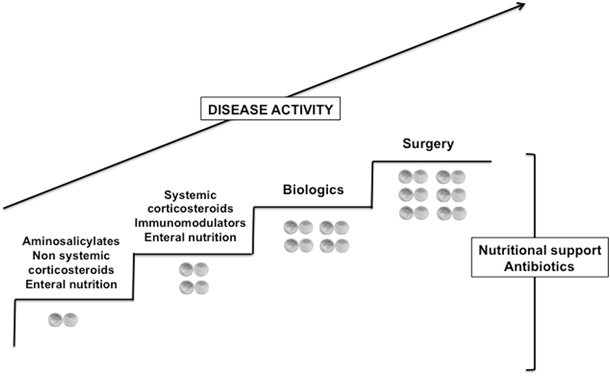

Fig. 28.3
Step-up therapeutic approach of pediatric Crohn’s disease
Conventional Therapy
Aminosalicylates
Aminosalicylates (5-ASA) are commonly used in the management of pediatric CD, although there are no randomized controlled trials evaluating their efficacy for the induction and maintenance of remission in children. Current data in adults do not support their use in ileal CD, showing any (or a slightly) improvement compared to placebo, thus their use in CD should not be supported [72].
Steroids
Conventional corticosteroids (CSs) are used for the induction of remission in moderate-to-severe CD. CSs are usually quickly weaned after the induction, due to their known adverse effects. Budesonide, an oral steroid preparation that is released in the distal ileum and proximal colon, can be used in patients with mild-to-moderate disease of those segments [73]. It has less systemic side effects than conventional steroids but is not completely without them, and quick withdrawal can lead to adrenal insufficiency [74].
Immunomodulators (Azathioprine, 6-Mercaptopurine, Methotrexate)
Thiopurines , comprising azathioprine and its active metabolite 6-mercaptopurine, are widely used maintenance agents in CD [75]. Their efficacy has been demonstrated in several trials for both induction and maintenance of remission in CD. However, due to their well-known slow onset of action (about 2–3 months), they are not used for induction of remission. Although the only pediatric prospective, multicenter, double-blind, placebo-controlled trial conducted in children reported 91 % of children receiving thiopurines to be in remission after 18 months of therapy [76], in routine clinical practice, a complete remission can be achieved in about 60 % of patients 1 year after beginning therapy [77]. Methotrexate is often regarded as a second-line immunomodulator in CD patients not responding or intolerant to thiopurines . Some data in adults have demonstrated its efficacy in inducing and maintaining remission [78]. To date, there have been no controlled trials of its use in pediatric CD, but reports from retrospective reviews and uncontrolled trials have shown good remission rates [79].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








