Patients with penile cancer who are proven to have negative inguinal lymph nodes have an excellent prognosis. Furthermore, patients with small-volume inguinal node involvement can often be cured by surgery alone. Lymphadenectomy has clear survival benefits for patients when applied to those with lymph node metastasis. However, the current morbidity of the standard technique of lymphadenectomy is an impediment to its universal application, and innovative strategies to reduce the morbidity of staging/treatment that do not compromise oncologic control must be developed and standardized. The optimal integration of multimodality therapy to improve survival in advanced disease will occur only through collaborative studies between centers with significant patient volume, which would be facilitated through the development of regional referral centers.
Penile cancer has a predictable pattern of spread in a stepwise fashion. Initially regional inguinal lymph node metastasis occurs, followed by pelvic nodal metastasis and then distant spread. Patients who are proven to have negative inguinal lymph nodes have an excellent prognosis. Furthermore, patients with small volume inguinal node involvement can often be cured by surgery alone. It seems rational then that lymphadenectomy would be standard in all patients; however, controversies arise due to the morbidity of the procedure. The difficulty in using purely clinical examination or imaging in selecting patients at risk for metastasis has led to divergent strategies such as watchful waiting versus sentinel lymph node biopsy versus limited dissections. However, new techniques are evolving that aim to effectively stage the inguinal region while minimizing morbidity. The prognosis for patients with advanced inguinal metastasis is dismal with surgery alone, and novel treatment strategies are clearly needed. This article describes current controversies and challenges in managing the inguinal region for patients with squamous penile cancer.
Objectives of lymphadenectomy
The main objective of lymphadenectomy is to provide accurate pathology-based staging and, where disease exists, to alter the natural history, clarify prognosis, and guide the future follow-up schedule. In cases with proven lymph node metastases, further therapy may be selected based on pathologic findings. Finally the provision of tissue, both from the primary tumor and from lymph nodes, is important for ongoing research.
Effect of Lymphadenectomy on Natural History of Penile Cancer
The greatest single predictor of survival in squamous cell carcinoma of the penis is the incidence and extent of lymph node involvement. In a prospective study the 3-year disease-specific survival of men with pathologic N0 disease was 100%. Also of note was that men found to have a single lymph node involved (N1 disease) had a 100% 3-year disease-specific survival without any adjuvant therapy. Presuming that the natural history of untreated micrometastatic lymph node disease become symptomatic, this result implies that surgery alone has a significant impact in early lymph node disease. Thus, a properly performed lymphadenectomy can cure some men with nodal metastasis. For more advanced nodal disease the number of positive lymph nodes predicts the 5-year overall survival as 75.6% for patients with between 1 and 3 nodes, 8.4% for 4 or 5 nodes, and 0% for those with more than 5 positive nodes. Other ominous features that threaten cure with surgery alone include the presence of extranodal extension and pelvic lymph node metastasis. However, a pelvic dissection can at times be beneficial, as demonstrated in some reports where 16% to 20% of patients with pelvic metastasis experienced long-term survival. Recently, a novel variable incorporating the number of positive lymph nodes/total nodes removed, termed the lymph node density (LND ), was described in penile cancer. The total lymph node count captures both the extent of dissection and the degree of processing by the pathologist. In this study TNM stage was compared with LND as prognostic indices of disease-specific survival. Median node counts for superficial inguinal lymph node dissection (ILND) were 8 to 10, for radical ILND 10 to 11, and for combined inguinal/ipsilateral pelvic node dissection 22 to 25. On univariate regression analysis, disease-specific survival was associated with pathologic N3 disease, extracapsular extension, the number of positive lymph nodes, and LND. As only 18 patients died during the follow-up period, full multivariate regression analysis was not possible. However, it was possible to sequentially compare 2 indices at a time to assess their relative importance. Comparing the various indices, when LND was incorporated into the model, the other variables including TNM stage and extranodal extension lost significance. An LND of greater than 6.7% had a hazard ratio of 14.48 of disease-specific mortality compared with an LND less than 6.7%. Also, when the group of patients with positive lymph nodes had LND values broken down into increasing tertiles, the hazard ratios of mortality were 1, 4.35, and 35.54, respectively. Of note, an additional finding of the study was that the number of negative lymph nodes removed among the lymph node–positive cohort was also a strong prognostic factor. This result would potentially suggest that microscopic metastases existed in this node-positive cohort and that a thorough dissection cleaned out metastases that were not detected via routine pathologic process. This novel concept requires independent validation in larger data sets to determine its routine clinical utility.
Early or Late Lymphadenectomy
The low rate of micrometastatic disease, along with the rarity of penile cancer in some countries and the morbidity of inguinal lymphadenectomy, may account for the practice of observation of men with invasive penile cancer and no palpable inguinal adenopathy. The lack of guidelines has meant that practice has varied considerably. Several articles have addressed the issue of early lymphadenectomy versus waiting for inguinal nodes to become palpable before offering surgery. There is no randomized study of immediate ILND versus surveillance, and as such a study is neither feasible nor ethical given our current understanding from studies provided below.
McDougal showed that patients who underwent lymphadenectomy for palpable disease had a 33% survival as compared with 84% for those with impalpable positive nodes. In another retrospective study from India, 28 patients underwent inguinal lymphadenectomy at either an early interval (mean of 1.7 months) or late interval (mean of 14 months) following surgery for the primary tumor. Although not randomized, the groups matched well for stage and grade. The 5-year cancer-specific survival rates for early and delayed ILND groups were 90.9% and 13.3%, respectively. Those undergoing delayed ILND had a significantly higher rate of extracapsular extension. One could argue, however, that these series also included patients in the “early group” whose lymph nodes were pathologically negative, and that they did not benefit from surgery but were exposed to the complications. This question was addressed in another study by Kroon and colleagues from the Netherlands, who compared the disease-specific survival of 20 patients who underwent an early therapeutic dissection and had proven positive nodes versus a group that underwent surgery subsequent to recurrence. Three-year disease-specific survival in the early group was 84% versus 35% in the delayed group ( P = .0017). This study clearly demonstrates that delayed lymphadenectomy is not a safe oncological practice for the majority of patients with clinically negative lymph nodes who exhibit “high-risk” features in their primary tumors. Further, it is now realized that the morbidity of a prophylactic dissection tends to be lower than palliative dissection performed for higher volume disease for which additional therapy is often required (ie, chemotherapy, radiation).
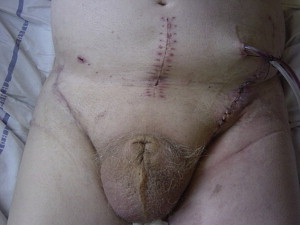
Although early lymphadenectomy is superior to delayed lymphadenectomy, performing bilateral ILND simultaneously with surgery for the primary lesion has several disadvantages. First, it requires accurate grading of the lesion preoperatively and may demand intraoperative frozen section to stage the lesion. Second, bilateral lymphadenectomy may affect the healing of the primary lesion because of lymphedema or infection. This situation is pertinent in cases where reconstructive techniques such as glansectomy and split-skin grafting are performed. Despite technical advances that preserve shaft length and function, chronic scrotal edema may lead to loss of a pendulous shaft, necessitating additional procedures ( Fig. 1 ). Despite these concerns, simultaneous ILND may be indicated in specific cases when compliance or comorbidity is an overriding factor.
Morbidity of Lymph Node Surgery
ILND sometimes requires lengthy skin incisions, creation of skin flaps beneath Scarpa’s fascia, and excision of lymphatic tissue with division of lymphatics. This procedure can have significant morbidity, and has been reviewed recently by Protzel and colleagues and Spiess and colleagues. The range of recorded morbidities for different approaches is summarized in Table 1 . Contemporary series show that the morbidity of ILND has decreased in recent years, with Bouchot and colleagues reporting 12% overall complications. Nonetheless, many centers have higher complication rates, and this has fueled very different strategies. The first strategy involves improving patient selection for ILND by performing dynamic sentinel node biopsy (DSNB), as described below. The other strategy for reducing the morbidity of ILND uses minimally invasive laparoscopic techniques, and has been addressed by 2 pioneering groups. These laparoscopic procedures have been called video endoscopic inguinal lymphadenectomy (VEIL) and endoscopic lymphadenectomy for penile cancer (ELPC). This strategy has the potential to reduce skin-related morbidity substantially (see Table 1 ), although the lymphatic complications may be similar to modified or superficial dissection.
| DSNB | VEIL/ELPC | Superficial Modified ILND | Radical ILND | |
|---|---|---|---|---|
| Range of patient #/series | 22–92 | 8–10 | 7–118 | 22–234 |
| Skin (%) | 0–13 | 0 | 0–4.5 | 7.5–61 |
| Infection (%) | 2.6–13 | 0 | 0–14.2 | 7.5–14.2 |
| DVT (%) | 0 | 0 | 0 | 0–12.1 |
| Seroma (%) | 1.3 | 0 | 12.1–26.3 | 5–13.8 |
| Edema (%) | 1.1–1.7 | 0 | 3–20 | 14.2–22.4 |
| Lymphocele (%) | 1.7–21.7 | 0–23 | 0–30 | 2.5–5.2 |
| Major (%) | 0–1.3 | 0 | 0–14 | 5–37.5 |
| Minor (%) | 6.6–39 | 20–23 | 6.8–36.8 | 45–54 |
Current strategies to select patients for prophylactic inguinal staging/therapeutic procedures
Imaging
The clinical staging of penile cancer is inaccurate, with significant under- and overstaging of inguinal nodes. In patients with impalpable nodes who undergo prophylactic ILND, micrometastatic disease is present in about 20% to 25%, whereas between 50% and 80% of palpable inguinal nodes harbor metastases. Radiological staging may miss micrometastatic disease. Three modalities have recently been evaluated in staging the inguinal region in patients with clinically negative nodes. Ultrasound-guided fine-needle aspiration cytology (FNAC) detected inguinal metastases in 9 of 23 (39%) patients with proven inguinal node metastases. Although the sensitivity was low, the investigators used this as a staging procedure whereby patients with positive findings underwent immediate lymphadenectomy and were spared DSNB (an estimated 11% patients were spared DSNB). Of note, FNAC has significant value among patients with palpable nodes, as Saisorn and colleagues reported a sensitivity of 93% in a recent study. The initial promise regarding 18 F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) in staging the inguinal region in penile cancer has been tempered by a recent report from Leijte and colleagues from the Netherlands. Among 42 patients with clinically negative inguinal nodes, metastases were found in 5 but PET/CT detected only 1 of these (ie, sensitivity = 20%). Of note, size of nodal metastases missed in the study ranged from 1 to 10 mm. PET/CT did pick up metastases that ranged from 20 to 60 mm. Thus its utility in detecting microscopic inguinal metastases remains unproven. PET/CT was valuable, however, in the detection of pelvic metastasis among patients with proven inguinal metastases. The use of lymphotrophic nanoparticles with magnetic resonance imaging had some promise in one small study among penile cancer patients; however, as of this date this procedure remains commercially unavailable.
Penile Cancer Staging Systems: Modifications to the Sixth Edition TNM Staging System
The TNM staging system is a widely accepted staging tool. However, deficiencies in the sixth edition TNM ( Table 2 ) of the American Joint Committee on Cancer (AJCC) were highlighted in a report of 513 cases treated over a 50-year interval at a single institute. The investigators described no difference in survival between stages T2 and T3 and nodal stages N1 and N2. Based on their own data, they recommended changes in the staging system (ie, the existing sixth edition TNM) with more meaningful prognostic stratification. This modified TNM system was relevant in that the variables examined were a part of routine clinical staging, in distinction to the sixth edition TNM, which is in essence a pathologic system.
| Sixth Edition | Seventh Edition |
|---|---|
| T – Primary tumor TX Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ Ta Noninvasive verrucous carcinoma T1 Tumor invades subepithelial connective tissue T2 Tumor invades corpus spongiosum or cavernosum T3 Tumor invades urethra or prostate T4 Tumor invades other adjacent structures | TX Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ Ta Noninvasive verrucous carcinoma (broad pushing invasion is permitted, destruction invasion is not) T1a Tumor invades subepithelial connective tissue without lymphovascular invasion and is not poorly differentiated T1b Tumor invades subepithelial connective tissue and either has lymphovascular invasion or is poorly differentiated T2 Tumor invades corpus spongiosum or corpus cavernosum T3 Tumor invades urethra T4 Tumor invades other adjacent structures |
| N – Regional lymph nodes NX Regional lymph nodes cannot be assessed N0 No evidence of lymph node metastasis N1 Metastasis in a single inguinal lymph node N2 Metastasis in multiple or bilateral superficial lymph nodes N3 Metastasis in deep inguinal or pelvic lymph nodes, unilateral or bilateral | Clinical stage, based on palpation and imaging: cNX Regional lymph nodes cannot be assessed cN0 No palpable or visibly enlarged inguinal lymph nodes cN1 Palpable mobile unilateral inguinal lymph node cN2 Palpable mobile multiple or bilateral inguinal lymph nodes cN3 Palpable fixed inguinal nodal mass or pelvic lymphadenopathy, unilateral or bilateral Pathologic stage, based on biopsy or surgical excision: pNX Regional lymph nodes cannot be assessed pN0 No regional lymph node metastases pN1 Metastasis in a single superficial, inguinal lymph node pN2 Metastasis in multiple or bilateral superficial inguinal lymph nodes pN3 Metastasis in deep inguinal or pelvic lymph node(s), unilateral or bilateral |
| M – Distant metastases MX Distant metastases cannot be assessed M0 No evidence of distant metastases M1 Distant metastases | M0 No distant metastasis M1 Distant metastasis, or lymph node metastasis outside the true pelvis |
On January 1, 2010, the seventh edition of the unified TNM staging for penile cancer became standard. This edition represents consensus between representatives of the AJCC and Union Internationale Contre le Cancer (UICC). It is the first alteration in the official TNM penile cancer staging since 1987 and includes significant changes:
- •
T1 is subdivided into T1a and T1b, based on lymphovascular invasion (LVI) and grade. This includes the practical division of T1 into high and low risk for selecting patients for ILND, when inguinal nodes are clinically impalpable.
- •
Invasion of the prostate has moved from T3 to T4, with T3 denoting urethral invasion only.
- •
There is provision for clinical and pathologic lymph node assessment. The distinction between superficial and deep inguinal lymph nodes has been eliminated.
- •
In the absence of nodal or metastatic disease, the new subdivision T1b becomes Stage II, while T1a remains Stage I.
- •
Any lymph node–positive disease is now at least Stage III.
Clinical and pathologic staging not only determines prognosis but forms the basis of integrating multimodal therapy in the management of advanced disease. These changes aim to clarify the management of cancer, facilitate meaningful comparison between cohorts, and support multi-institutional research. Future studies should compare the prognostic value of both the seventh edition TNM and that proposed by Leitje and colleagues using large data sets to determine the optimal variables that best stratify patient prognosis.
Impact of Primary Tumor Histologic Features on Predicting Occult Nodal Metastasis
Patients with primary tumors exhibiting carcinoma in situ or verrucous carcinoma have little or no risk for metastasis. Only 2 cases of metastasis in association with carcinoma in situ have been reported, and none of 47 cases of penile verrucous carcinoma has been shown to metastasize. Thus, patients with both Tis and Ta penile cancer are included in the low-risk group for inguinal metastases.
In contrast, patients with corporal invasion (TNM stage pT2) in the penile tumor exhibit a high risk for metastasis. The average risk for inguinal metastasis among 225 patients in 7 different series was 59%.
Stage T1 penile cancers exhibit involvement of the subepithelial connective tissue only and lack involvement of the corpus spongiosum, corpora cavernosa, or urethra. Similarly staged tumors historically have been associated with a 4% to 14% incidence of nodal metastasis. Theodorescu and colleagues noted one exception to this relatively low rate of metastatic disease; 58% of patients (14 of 24) with pT1 primary tumors and initially negative nodes on clinical assessment subsequently developed inguinal nodal metastases. These data suggest that other variables present within the penile cancers of the cohort of patients studied (ie, tumor grade and presence of vascular invasion) may have modified the effect of tumor stage on metastasis.
Several investigators have evaluated the risk of nodal metastasis for TNM stage T1 lesions according to tumor grade. Among 73 patients with T1, grade 1 or grade 2 primary tumors, metastasis occurred in only 5 patients (7%). Recent data from Naumann and colleagues, however, suggested that among stage T1 grade 2 tumors specifically, the risk of metastases could be higher than previously described. Recently, Hughes and colleagues reported on a larger 2-center experience where, among 105 node-negative patients at presentation, 9 (9%) exhibited lymph node metastases at surgery or on follow-up. Thus the incidence overall was much lower in this cohort.
Ficarra and colleagues developed the first penile cancer nomogram using data from 175 patients. Based on tumor thickness and growth pattern, patients with T1 grade 2 tumors exhibited metastatic rates from 5% to 20%. Thus Grade 2 tumors represent a heterogeneous group wherein the histologic criteria used to describe grade 2 and the presence or absence of other poor prognostic features ultimately determines prognosis. In this regard, the European Association of Urology Guidelines (EAU ) assigned patients with T1 Grade 2 tumors to the intermediate-risk category wherein the risk of lymph node metastasis is greater than 16% (low risk) and less than 68% (high risk) ( Table 3 ).
| Group | Primary Tumor |
|---|---|
| Low risk | Tis, Ta, T1 G1 |
| Intermediate risk | T1 G2 |
| High risk | T1 G3, any T2 or greater |
The presence of vascular invasion as a prognostic indicator of inguinal lymph node metastasis in squamous penile cancer is now evident. Lopes and colleagues studied the prognostic value of lymphatic invasion in 146 patients with penile cancer. In a univariate analysis, clinical nodal stage, tumor thickness, lymphatic and venous embolization, and urethral infiltration were all associated with lymph node metastasis. However, subsequent to multivariate analysis, only venous and lymphatic invasion remained significant predictors for positive lymph nodes. Data from the University of Texas M.D. Anderson Cancer Center revealed that vascular invasion was absent in all patients with T1 tumors. These patients were also lymph node negative at surgery. In contrast, patients with stage pT2 primary tumors exhibited nodal metastasis in 75% of cases (15 of 20) when vascular invasion was present but in only 25% of cases (3 of 12) when it was absent.
Taking this a step further and including the variables of tumor thickness, growth pattern, grade, venous/lymphatic invasion, corpus spongiosum, or cavernosum involvement, urethral involvement, and palpable lymph nodes, Ficarra and colleagues developed their nomogram predicting inguinal lymph node involvement. The most important variables were venous/lymphatic invasion and the presence of palpable nodes in multivariate analysis. The concordance index of the nomogram was very good, at 0.876. However, external validation of the nomogram is pending at this time. Table 3 depicts variables associated with low, intermediate, and higher risks of metastasis. The International Consultation on Penile Cancer was held in Santiago, Chile in 2008. Consensus recommendations from in international panel of 38 investigators were reported (ICUD-Penile). Fig. 2 reports risk groups, metastatic rates, and management strategies from the EAU, ICUD-penile, and the literature. In general, all seem to agree that patients with clinically negative inguinal nodes in the high-risk group should have an inguinal staging procedure performed as a primary recommendation. Similarly, in the low-risk group these same patients who are felt to be compliant are offered surveillance. Among the intermediate group the EAU guidelines recommend observation for those patients with T1 superficial tumors and no vascular invasion. Otherwise, with all other pathologic findings an inguinal staging procedure is recommended. The ICUD-penile panel recommended that either observation or an inguinal staging procedure is appropriate for intermediate-risk patients as long as they are informed of risks and benefits.
Current strategies to select patients for prophylactic inguinal staging/therapeutic procedures
Imaging
The clinical staging of penile cancer is inaccurate, with significant under- and overstaging of inguinal nodes. In patients with impalpable nodes who undergo prophylactic ILND, micrometastatic disease is present in about 20% to 25%, whereas between 50% and 80% of palpable inguinal nodes harbor metastases. Radiological staging may miss micrometastatic disease. Three modalities have recently been evaluated in staging the inguinal region in patients with clinically negative nodes. Ultrasound-guided fine-needle aspiration cytology (FNAC) detected inguinal metastases in 9 of 23 (39%) patients with proven inguinal node metastases. Although the sensitivity was low, the investigators used this as a staging procedure whereby patients with positive findings underwent immediate lymphadenectomy and were spared DSNB (an estimated 11% patients were spared DSNB). Of note, FNAC has significant value among patients with palpable nodes, as Saisorn and colleagues reported a sensitivity of 93% in a recent study. The initial promise regarding 18 F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) in staging the inguinal region in penile cancer has been tempered by a recent report from Leijte and colleagues from the Netherlands. Among 42 patients with clinically negative inguinal nodes, metastases were found in 5 but PET/CT detected only 1 of these (ie, sensitivity = 20%). Of note, size of nodal metastases missed in the study ranged from 1 to 10 mm. PET/CT did pick up metastases that ranged from 20 to 60 mm. Thus its utility in detecting microscopic inguinal metastases remains unproven. PET/CT was valuable, however, in the detection of pelvic metastasis among patients with proven inguinal metastases. The use of lymphotrophic nanoparticles with magnetic resonance imaging had some promise in one small study among penile cancer patients; however, as of this date this procedure remains commercially unavailable.
Penile Cancer Staging Systems: Modifications to the Sixth Edition TNM Staging System
The TNM staging system is a widely accepted staging tool. However, deficiencies in the sixth edition TNM ( Table 2 ) of the American Joint Committee on Cancer (AJCC) were highlighted in a report of 513 cases treated over a 50-year interval at a single institute. The investigators described no difference in survival between stages T2 and T3 and nodal stages N1 and N2. Based on their own data, they recommended changes in the staging system (ie, the existing sixth edition TNM) with more meaningful prognostic stratification. This modified TNM system was relevant in that the variables examined were a part of routine clinical staging, in distinction to the sixth edition TNM, which is in essence a pathologic system.
| Sixth Edition | Seventh Edition |
|---|---|
| T – Primary tumor TX Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ Ta Noninvasive verrucous carcinoma T1 Tumor invades subepithelial connective tissue T2 Tumor invades corpus spongiosum or cavernosum T3 Tumor invades urethra or prostate T4 Tumor invades other adjacent structures | TX Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ Ta Noninvasive verrucous carcinoma (broad pushing invasion is permitted, destruction invasion is not) T1a Tumor invades subepithelial connective tissue without lymphovascular invasion and is not poorly differentiated T1b Tumor invades subepithelial connective tissue and either has lymphovascular invasion or is poorly differentiated T2 Tumor invades corpus spongiosum or corpus cavernosum T3 Tumor invades urethra T4 Tumor invades other adjacent structures |
| N – Regional lymph nodes NX Regional lymph nodes cannot be assessed N0 No evidence of lymph node metastasis N1 Metastasis in a single inguinal lymph node N2 Metastasis in multiple or bilateral superficial lymph nodes N3 Metastasis in deep inguinal or pelvic lymph nodes, unilateral or bilateral | Clinical stage, based on palpation and imaging: cNX Regional lymph nodes cannot be assessed cN0 No palpable or visibly enlarged inguinal lymph nodes cN1 Palpable mobile unilateral inguinal lymph node cN2 Palpable mobile multiple or bilateral inguinal lymph nodes cN3 Palpable fixed inguinal nodal mass or pelvic lymphadenopathy, unilateral or bilateral Pathologic stage, based on biopsy or surgical excision: pNX Regional lymph nodes cannot be assessed pN0 No regional lymph node metastases pN1 Metastasis in a single superficial, inguinal lymph node pN2 Metastasis in multiple or bilateral superficial inguinal lymph nodes pN3 Metastasis in deep inguinal or pelvic lymph node(s), unilateral or bilateral |
| M – Distant metastases MX Distant metastases cannot be assessed M0 No evidence of distant metastases M1 Distant metastases | M0 No distant metastasis M1 Distant metastasis, or lymph node metastasis outside the true pelvis |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





