Fig. 9.1
Correspondence between endo-ultrasonographic (a) and anatomical (b) layers of the GI wall (white arrows). P probe
Endo-ultrasonography is also able to provide high-resolution images of the organs and structures which are adjacent to the GI wall. Therefore, EUS can adequately evaluate locoregional lymph nodes (N parameter). A size larger than 10 mm; a round shape, well-demarcated boundaries; and echo-poor, homogeneous texture represent EUS criteria suggestive of lymph node involvement (Fig. 9.2). Malignancy is almost certain only when all of the abovementioned features are recognized at the same time. Diagnostic yield of EUS can be significantly improved by performing a FNAB under EUS guidance.
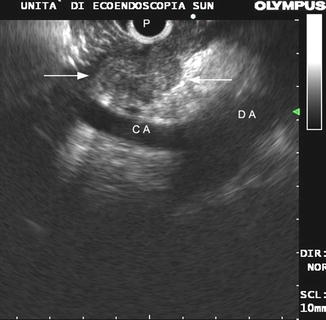

Fig. 9.2
Malignant lymph node (between arrows). All typical endosonographic features are shown (size larger than 10 mm, round shape, well-demarcated boundaries and echo-poor, homogeneous texture). P probe, CA celiac artery, DA descending aorta
9.3 Endo-ultrasonography for the Diagnosis of Anastomotic Recurrences: Early Results
Preoperative locoregional staging of the gastrointestinal tract cancers was one of first and most successful applications of EUS. Therefore, since the late 1980s, endo-ultrasonography has also been proposed for the diagnosis of anastomotic recurrences after surgical resection of gastrointestinal tumors.
In 1989, Lightdale et al. [1] first described the use of radial EUS after radical surgery for esophageal or gastric cancer in 40 patients with a clinical suspicion of local recurrence. EUS correctly identified recurrent malignancy in 23 out of 24 patients in whom a local recurrence was then proven, showing an overall sensitivity of 95 % and a specificity of 80 %. In six cases EUS was the only technique that diagnosed the recurrence.
In 1986 Hildebrand et al. [2] published the first study on rectal cancer. They reported a local recurrence in 22 patients who had undergone surgical resection, within a 3-year observation period. Transrectal ultrasound (TRUS) identified all the lesions (sensitivity 100 %) and was the only method that allowed for the diagnosis in nine cases (41 %). Also in a series by Beynon et al. [3], TRUS detected all 22 recurrences which occurred in a group of patients treated by radical surgery. However, in 19 of the 22 patients, the recurrence was also identified by digital examination or proctoscopy, so that only in 3 of the 22 patients (13.6 %), the diagnosis was based exclusively on ultrasonography.
The abovementioned results confirmed EUS as a very promising technique for the early diagnosis of anastomotic recurrences but were not sufficient to establish its clinical impact and to justify the inclusion of endo-ultrasonography in follow–up protocols for the surveillance of patients who had undergone surgical resection of gastrointestinal tract cancer.
9.4 Criteria for Endo-ultrasonographic Diagnosis of Tumor Recurrence
Endo-ultrasonographic imaging of gastrointestinal anastomoses can vary depending on several factors.
One of the most important is the interval between the surgical operation and the EUS examination. The longer the time is, the lesser the postoperative changes at the level of the anastomosis are. EUS imaging also depends on the surgical technique which has been employed in performing the anastomosis. The effects of local surgical complications can also considerably affect EUS imaging of anastomosis. So it is very important to know if anastomotic leakage, abscess, or fistula had occurred in the early postoperative period (Fig. 9.3).
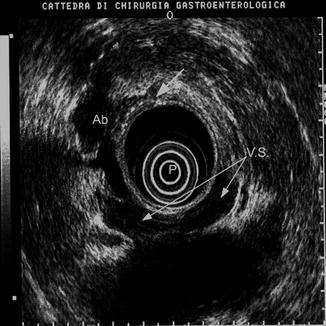

Fig. 9.3
Perirectal abscess (Ab) following a leakage of the anastomosis (white arrow). EUS appearance is an echo-poor lesion which can mimic a tumor recurrence. P probe, VS vesiculae seminales
Usually, EUS imaging of a gastrointestinal anastomosis, if evaluated within the first 6 months following surgical resection, shows a significant thickening of the wall, with circumferential hypertrophy of the third and the fourth layer (submucosa and muscularis propria, respectively) related to postoperative changes (Fig. 9.4). Hypertrophy of the submucosa generally disappears in a short time, whereas the circumferential thickness of the Mp. may last for even several years [4]. A previous radiotherapy [5] or manual anastomosis [6] can cause a persistent broadening of the wall. Sometimes typical five-layer wall structure is no longer recognized, and it is replaced by a mixed echoic tissue, sometimes with a “pseudo lamellar” appearance. Especially in the case of rectal anastomosis, the outer margin of the wall is often irregularly shaped along the whole circumference, but it is always demarcated from the surrounding tissues (Fig. 9.5) [7].
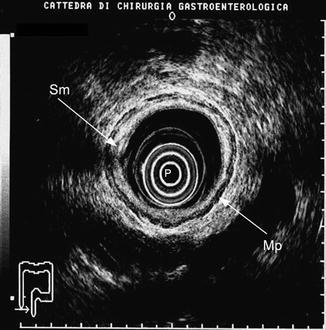
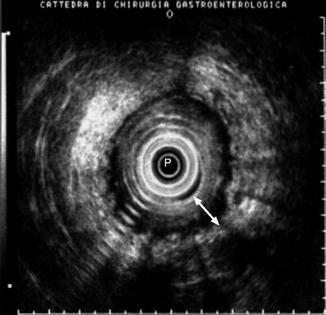

Fig. 9.4
A colorectal anastomosis evaluated within the first 6 months after surgical resection. Note the hypertrophy of the submucosa (Sm). Mp muscularis propria

Fig. 9.5
Rectal anastomosis of a patient who had undergone radiotherapy. Note the thickening of the wall (white arrow) and the irregularity of the outer margin which is well demarcated from the surrounding tissues. P probe
Once postoperative changes have disappeared, it can be difficult to even identify the site of the anastomosis [8]. A mechanical anastomosis is almost always visible because of the staples which appear as a circumferential line of small, bright echoes without a shadow [9] (Fig. 9.6).
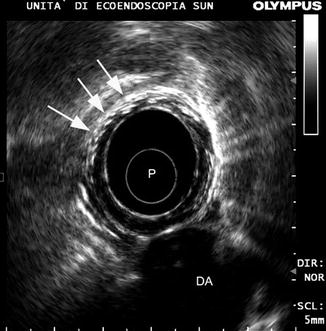

Fig. 9.6
Mechanical esophageal anastomosis. The staples produce a circumferential line of small bright echoes without a shadow (white arrows). P probe, DA descending aorta
The so-called central recurrences, which appear as a focal echo-poor inhomogeneous thickening originating at the site of the anastomosis (Fig. 9.7), are quite rare, mainly developing as a consequence of an inadequate surgical resection of the primary tumor and a microscopic tumor infiltration of the surgical margins. Apart from technical errors, these types of recurrences can also occur depending on the location of the primary tumor, for instance, in the case of low rectal cancers treated by preserving sphincters surgical resections or in the case of cancers of the cardia involving the distal part of the esophagus treated with a gastrectomy plus distal esophagectomy by a transhiatal approach.
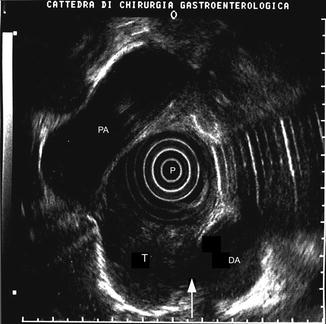

Fig. 9.7
A “central” recurrence (T) at the site of esophageal anastomosis. Note the adherence (arrow) with the wall of the descending aorta (DA). PA pulmonary artery
In truth, most of the anastomotic recurrences have an extra-luminal development or are located out of the wall, so they could be better defined as “locoregional recurrences.” With regard to rectal cancer, up to 80 % of the tumor recurrences develop in the perianastomotic area or in the pelvic space (Fig. 9.8) [10].
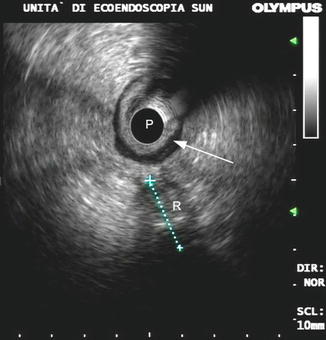

Fig. 9.8
A pelvic recurrence (R) originating out of the rectal wall (arrow). P probe
A local recurrence usually appears at the endo-ultrasonographic evaluation as an irregularly shaped echo–poor mass with a heterogeneous texture involving from outside the gastrointestinal wall (Fig. 9.9). However, less typical findings may also be found. De Witt et al. [11] reported that 16 out of 21 tumor recurrences (76 %) were hypoechoic at EUS, but remnants were hyperechoic or had a mixed echo texture. The presence of lesions with echo-free areas usually suggests the diagnosis of postoperative seroma or hematoma, but it is not infrequent to find perirectal recurrence with large fluid components (Fig. 9.10).
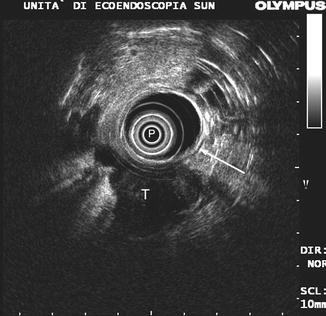
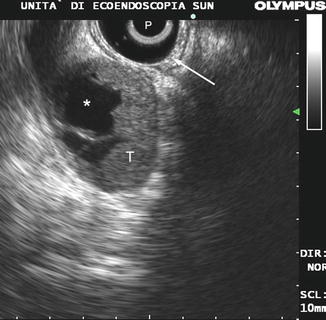

Fig. 9.9
A local recurrence after surgical resection of rectal cancer. Note the irregularly shaped echo-poor mass (T) involving from outside the rectal wall (arrow). P probe

Fig. 9.10




Local recurrence of rectal cancer (T) with a fluid component located close to the rectal wall at the level of the anastomosis (arrow). P probe
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








