The most widely used continuous renal replacement therapies (CRRT) for the treatment of critically ill patients in renal failure are continuous hemodialysis and hemodiafiltration. Two prolonged intermittent renal replacement therapies (PIRRT), sustained low-efficiency hemodialysis and sustained low-efficiency hemodiafiltration, are also quite popular. Continuous hemofiltration and slow continuous ultrafiltration are used, but less commonly.
I. NOMENCLATURE. In the Handbook, we abbreviate continuous hemodialysis as C-HD, whether it is applied using an arteriovenous (AV) or venovenous access. Similarly, continuous hemofiltration is abbreviated as C-HF, and their combination, slow continuous hemodiafiltration, is C-HDF. Historically, one would insert “AV” or “VV” after the letter “C” to specify that the therapy was given using either an AV or a venovenous access, giving CAVHD or CVVHD (hemodialysis), CAVH or CVVH (hemofiltration), and CAVHDF or CVVHDF (hemodiafiltration); but today, most treatments are given using a venous catheter-based access, and so use of the “VV” has become superfluous. Slow continuous ultrafiltration is abbreviated as SCUF, and sustained low-efficiency hemodialysis and hemodiafiltration are abbreviated as SLED and SLED-F, respectively. SLED and SLED-F are collectively grouped as forms of prolonged intermittent renal replacement therapy, or PIRRT. Conventional intermittent treatment is called IHD (intermittent hemodialysis) or, more generally, IRRT (intermittent renal replacement therapy), given that the intermittent treatment being given is not always hemodialysis.
A.
What are the differences among C-HD, C-HF, and C-HDF? Each of these procedures involves slow, continuous passage of blood, taken from either an arterial or a venous source, through a filter.
Table 15.1 shows a comparison of these techniques.
1.
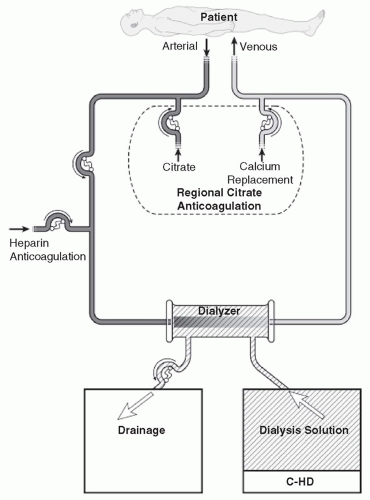
Continuous hemodialysis (C-HD). In C-HD (
Fig. 15.1), dialysis solution is passed through the dialysate compartment of the filter continuously and at a slow rate. In C-HD, diffusion is the primary method of solute removal. The amount of fluid that is ultrafiltered across the membrane is low (usually about 3-6 L per day) and is limited to excess fluid removal.
2.
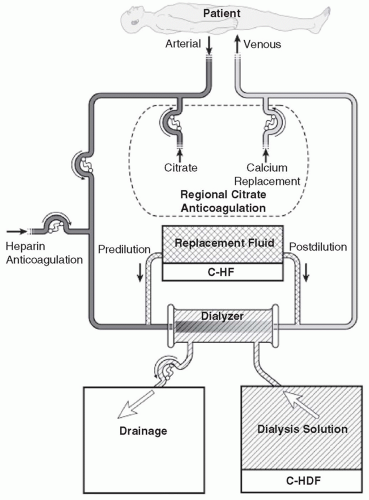
Continuous hemofiltration (C-HF). In C-HF (
Fig. 15.2), dialysis solution is not used. Instead, a large volume (about 25-50 L per day) of replacement fluid is infused into either the inflow or the outflow blood line (predilution or postdilution mode, respectively). With C-HF, the volume of fluid that is ultrafiltered across the membrane is the sum of replacement fluid and excess fluid removed, and so is much higher than with C-HD.
3.
Continuous hemodiafiltration (C-HDF). This (
Fig. 15.2) is simply a combination of C-HD and C-HF. Dialysis solution is used, and replacement fluid is also infused into either the inflow or the outflow blood line. The daily volume of fluid that is ultrafiltered across the membrane is equal to the replacement fluid infused plus the net volume removed. Usually, the
replacement fluid volume with C-HDF is about half that used with C-HF, but the total effluent volume (replacement fluid + dialysis solution + excess fluid volume removed) with C-HDF is similar to that with C-HF, where effluent volume is the sum of replacement fluid and excess fluid volume only.
4. Slow continuous ultrafiltration (SCUF). The setup is similar to that for C-HD and C-HF, but neither dialysis solution nor replacement fluid is used. Daily ultrafiltered fluid volume across the membrane is low (usually about 3-6 L per day), similar to C-HD.
B.
Sustained low-efficiency dialysis and hemodiafiltration (SLED and SLED-F). SLED is a form of IHD using an extended (6- to 10-hour) session length and reduced blood and dialysate flow rates. Typically, blood flow rates (BFRs) are about 200 mL/min and dialysate flow rate is 100-300 mL/min. Regular hemodialysis equipment can be used as long as low blood and dialysate flow rates are supported; a software update may be required with certain dialysis machines to provide the lower rates. The same machine used for IHD during the day often can be used for SLED during the night, and hemodialysis nurses can easily be trained to perform SLED, offering some economy of staff instruction. SLED allows units where CRRT equipment or personnel are unavailable or limited to offer a treatment modality that should achieve similar benefits as CRRT. SLED-F requires additional infusion of replacement fluid unless replacement fluid can be made from dialysis solution online by the dialysis machine (
Marshall, 2004).
II.
CLINICAL INDICATIONS FOR CRRT VERSUS INTERMITTENT RENAL REPLACEMENT THERAPY. The potential advantages of the various CRRT procedures as well as SLED are listed in
Table 15.2. They include a lower rate of fluid removal as well as enhanced control of azotemia when compared with standard IHD. Despite the seemingly obvious advantages of slow continuous therapies, there has been no evidence from several randomized trials that, in the acute renal failure setting, use of CRRT offers a survival advantage over IHD (Rabindranath, 2007). However, most trials excluded the sickest patients from treatment with conventional
IHD. The 2012 KDIGO Guidelines for acute kidney injury (AKI) suggest (level 2B evidence) that clinicians use CRRT rather than standard IRRT for hemodynamically unstable patients, and also suggest using CRRT to treat AKI patients with acute brain injury or other causes of increased intracranial pressure or generalized brain edema (
KDIGO AKI, 2012). However, the guidelines recognize that the use of prolonged IRRT therapies such as SLED or SLED-F may be as useful to treat hemodynamically unstable patients as CRRT, but point to the paucity of outcomes trials
comparing CRRT with prolonged IRRT. Some early comparisons (
Van Berendoncks, 2010;
Marshall 2011) suggest that outcome results with prolonged IRRT are similar to those of CRRT, and that considerable cost savings are achieved with prolonged IRRT.
III. TRAINING AND EQUIPMENT COSTS. The use of continuous procedures requires an effort on the part of nursing staff in the ICU to become familiar with the procedures. In units with high staff turnover rates and in units where continuous therapies are done infrequently, use of prolonged intermittent therapies such as SLED may be a more practical option. However, in high-volume units where continuous therapies are a common part of the dialysis armamentarium, use of such therapies can aid in the fluid, solute, and nutritional management of the most challenging patients.
IV. DIFFERENCES AMONG C-HD, C-HF, AND C-HDF IN CLEARANCE OF SMALL-AND LARGE-MOLECULAR-WEIGHT SOLUTES
A. Solute clearance with C-HD. In C-HD, where the BFR is 150-200 mL/min or more, and dialysate flow rate typically is 25-30 mL/min, clearance of urea and other small molecules is determined primarily by the dialysis solution flow rate. As a rule of thumb, BFR in C-HD should be at least three times the dialysate flow rate. At this slow BFR and high blood-to-dialysate flow ratio, the outflow dialysate is almost 100% saturated with urea and other small-molecular-weight (MW) solutes. Urea clearance can thus be simply estimated by the effluent volume, which includes the volume of dialysis solution used plus any excess fluid removed.
The standard dialysis solution inflow rate is now about 20-25 mL/kg per hour. In a 70-kg individual, this translates into a flow rate of 23-29 mL/min. If we assume a flow rate of 26 mL/min and 100% saturation, this will deliver a urea clearance of 26 mL/min or about 37 L per day, and if we add 3 L per day of excess fluid removal, this gives a daily effluent volume and urea clearance of 37 + 3 = 40 L. In terms of urea kinetics, this 40 L can be thought of as the familiar (
K ×
t) measure of clearance. For a patient with a urea distribution volume of 40 L, such a prescription would translate to a daily
Kt/V of 40/40 = 1.0 or about 7.0 per week. This compares favorably with an equivalent weekly
Kt/V urea delivered by thrice-weekly IHD of about 2.7 (see
Chapter 3 to find out how equivalent weekly
Kt/V urea is calculated).
B.
Solute clearance with C-HF. C-HF is a purely convection-based blood cleansing technique. As blood flows through the hemofilter, a transmembrane pressure gradient between the blood compartment and the ultrafiltrate compartment causes plasma water to be filtered across the highly permeable membrane. As the water crosses the membrane, it sweeps along with it (nonprotein-bound) small and large molecules (pore size permitting) and thus leads to their removal from the blood. The removed ultrafiltrate is replaced
by a balanced electrolyte solution infused into either the inflow (predilution) or the outflow (postdilution) line of the hemofilter. Typically, about 20-25 mL/kg per hour of replacement fluid is infused. The filter outflow or “drainage fluid” is nearly 100% saturated with urea when postdilution mode is used.
1. Filtration fraction. This is the fraction of plasma flowing through the hemofilter that is removed. Filtration fraction can be calculated as the ultrafiltration rate divided by the plasma flow rate. The latter is simply BFR × (1 – Hct). For example, if the BFR is 150 mL/min and the Hct is 33%, the plasma flow rate will be 0.67 × 150 = 100 mL/min. If the UF rate is 25 mL/min, then the filtration fraction is 25/97, or about 25%. The rule of thumb is to keep the filtration fraction at 25% or lower to avoid overconcentration of red cells and plasma proteins in the hemofilter. Overconcentration results in fouling of the membrane pores, which can impair UF efficiency and lower the sieving coefficient, and overconcentration can also increase the likelihood of clotting. To avoid overconcentration and keep filtration fraction below 25%, when a high replacement fluid infusion rate in postdilution mode is desired, the BFR needs to be increased above the usual 150 mL/min.
2. Predilution mode. Another way to keep the filtration fraction from increasing is to use predilution mode. With predilution, there is slight lowering of the urea concentration of ultrafiltrate (usually 80%-90% of the corresponding plasma value), but this is outweighed by the ability to deliver an increased replacement solution infusion rate, enhancing overall middle-molecule clearances. We recommend using predilution whenever it is desirable to remove more than 25 L per day. Predilution is also performed if the baseline blood viscosity is relatively elevated (e.g., if the hematocrit is >35%). A combination of pre- and postdilution has been advocated by some practitioners.
3. Calculating the dilutional effects of predilution mode. As an example, assume that the replacement fluid infusion rate is 25 mL/min and BFR is 150 mL/min. The amount of dilution of waste products in the blood entering the filter will be 25/(150 + 25) = 14%. Assuming that 35 L per day of replacement fluid is used and that 5 L per day of excess fluid is removed, daily effluent volume will typically be about 40 L per day. In postdilution mode, (K × t) t will be 40 L. In predilution mode, (K × t) will be perhaps 15% less, 34 L, and so, assuming V = 40 L, then daily Kt/V with C-HF will be about 40/40 = 1.0 (postdilution) or 34/40 = 0.85 (predilution).
C.
Urea clearance with C-HDF. With C-HDF, the sum of the dialysis solution flow rate, replacement fluid infusion rate, and removal of excess fluid usually is set at a level similar to the outlet flow rate in C-HD or postdilution C-HDF. The clearance calculations are similar to those discussed above. The clearance of
small molecules with C-HDF is similar to that with C-HD and C-HF when the daily effluent volumes are comparable.
D. Small- versus middle-molecular-weight solute removal with C-HF versus C-HD. With C-HD, the outflow dialysate is not as highly saturated with larger-MW substances that diffuse slowly in solution and thus have a lower rate of diffusive transfer across the dialyzer membrane. In contrast, with C-HF, the plasma ultrafiltrate is almost completely saturated with both low- and middle-MW solutes, because the convective removal rates of small- and larger-MW solutes are similar. Hence, C-HF is more efficient than C-HD in terms of larger-MW toxin removal, including peptides, certain antibiotics, and vitamin B12. The theoretical advantage of C-HF is technically demanding to realize, as it can be challenging to ultrafilter >25 L from patients who cannot deliver the high BFRs required to prevent overconcentration. Also, fluid balancing becomes critical when the replacement fluid infusion rate is high. With high-volume C-HF, any slowing of the BFR will result in transient hemoconcentration in the hemofilter, with attendant risk of clotting. On the other hand, it is easy to perform C-HD using dialysis solution flow rates of 50 L per day. For this reason, in daily practice, C-HD tends to be the more popular therapy, and if enhanced removal of middle molecules is desired, a replacement fluid component is added (C-HDF).
1.
Filter surface area and clearance of larger-MW substances: One in vitro study of clearance of larger-MW substances by C-HF versus C-HD with two different-sized filters (0.4 vs. 2.0 m
2) came up with some counterintuitive results: with the larger membrane, the clearance of large-MW substances was identical with C-HD and C-HF, and with the smaller (0.4-m
2 membrane), clearance of large-MW substances was actually worse with C-HF than with C-HD (
Messer, 2009). The proposed explanation was increased protein fouling of the smaller membrane in C-HF mode. These results suggest that using a high replacement fluid flow rate with a small hemofilter may not be an efficient way to increase removal of larger, middle molecules.
V. VASCULAR ACCESS
A.
Venovenous blood access. Vascular access is obtained using a dual-lumen cannula inserted into a large (internal jugular or femoral) vein. The subclavian vein can be used but is not the site of first choice. See
Chapter 7. The 2012 KDIGO AKI guidelines recommend using uncuffed venous catheters for CRRT (5.4.1). The level of evidence for this is weak (2D). The rationale is that insertion of an uncuffed catheter is easier, that the need for a cuffed catheter might sometimes delay initiation of therapy, and that the average duration of CRRT is only 12-13 days (
KDIGO, 2012). One study (
Morgan, 2012) compared use of longer (20-24 cm) soft, silicone temporary catheters for CRRT, targeting placement of the catheter tip near the right
atrium, versus shorter (15-20 cm) catheters targeting placement of the catheter tip in the superior vena cava; the longer catheters were associated with longer filter life and improved dose of therapy. In another study looking at the success rate of CRRT achieved by femoral venous access, filter longevity averaged 15 hours when the venous catheter was inserted on the right side versus 10 hours when the left femoral vein was used (
Kim, 2011). The mechanism of the advantage of right-sided femoral catheters was not clear.
B. Arteriovenous blood access. One can cannulate a large artery, usually the femoral artery, and propel blood through the extracorporeal circuit by using the patient’s own arterial pressure instead of a pump. Blood is returned via any large vein. Use of AV blood access for CRRT is no longer widely practiced. There is risk of damage to the femoral artery with possible distal limb ischemia, plus AV access will often not deliver high enough blood flows to be able to support the more intensive CRRT therapies in common use today. However, CRRT using AV access may be lifesaving in situations where a mass catastrophe has occurred (e.g., earthquake with renal injury due to rhabdomyolysis) and electrical power sources are unreliable, because blood flow is then driven by a patient’s own blood pressure and ultrafiltration is adjusted by using gravity (or clamp) via the height of the effluent collection container. For a detailed description of CRRT using an AV access, please refer to the third edition of the Handbook.
C. Catheter changes: Scheduled changes versus changing only when clinically indicated. CRRT catheters should be changed only when clinically indicated; catheters should not be changed according to some predetermined schedule in the hopes of minimizing the rate of catheter sepsis. The practice of routine, scheduled catheter changes, once popular, is not recommended by the Centers for Disease Control and Prevention (CDC), and studies do not support this approach.
VI. CRRT FILTERS. The terms “hemofilter” and “dialyzer” are used interchangeably in this chapter. Hemofilters have only one outlet in the housing, making use of dialysis solution impossible. Dialyzers have a second port. Dialyzers used for CRRT should have high water permeability, and so will be in the “high-flux” category. Some of the early filters designed primarily for C-HF had excellent water permeability and convective solute clearance, but had poor diffusive clearance when used for C-HD; there was poor optimization of contact between dialysis solution and all parts of the membrane in these filters. Currently used CRRT filters allow urea in the blood compartment of the filter to equilibrate promptly with dialysate, making them suitable for both C-HF and C-HD.
A.
Filter surface area and size. The size of the filter should take the BFR into account. When large filters are used at low BFRs, the risk of clotting may be increased, as such filters will have been designed for much higher BFRs. Flow velocity through each
fiber will be slow. Furthermore, the permeation of the fiber bundle by dialysis solution may be suboptimal with large dialyzers designed to be used at high dialysate flow rates. On the other hand, larger dialyzers can be used at higher BFRs such as those in some higher-efficiency SLED protocols, in order to maximize middle-molecule solute clearances. The study by
Messer et al. (2009) described above also suggests that use of a larger-size filter might be considered when removal of larger-MW solutes is desired and a high replacement fluid rate is to be used.
VII. DIALYSATES AND REPLACEMENT SOLUTIONS. CRRT fluids come premixed as commercially prepared sterile solutions. They are typically packaged in 2.5-L or 5-L bags; in some cases fluids are supplied in bags with two compartments that need to be mixed immediately prior to use.
A.
Composition. Table 15.3 lists the composition of some commonly available commercially prepared solutions for CRRT.
1. Buffers. Solutions contain either lactate or bicarbonate.
a. Lactate-based solutions. Pure lactate-based replacement fluid usually contains 40-46 mM of lactate. Lactate-based solutions effectively correct metabolic acidosis in most patients. Lactate is metabolized on a 1:1 molar basis to bicarbonate, but in practice, the dialysis solution lactate concentration needs to be higher than dialysis solution bicarbonate to effect similar degrees of correction of acidosis.
b. Bicarbonate-based solutions. Bicarbonate-containing bags are sold as two-compartment systems, similar to those used to prepare bicarbonate-containing dialysis solution for peritoneal dialysis. Bicarbonate is the buffer of choice, and total base concentrations are typically 25-35 mM. Some solutions contain a small amount (3 mM) of lactate, left over from lactic acid used to acidify the final solution. There is no evidence that this small amount of lactate contributes to hyperlactatemia.
When a high dialysis solution or replacement solution flow rate (e.g., >30 mL/kg/hour) is prescribed, use of lower bicarbonate solutions may help prevent metabolic alkalosis. Lower bicarbonate concentration solutions or bicarbonate-free solutions are also indicated when using regional citrate anticoagulation, because citrate is metabolized to bicarbonate by the liver.
c.
When high-lactate solutions should be used with caution: Use of solutions using lactate as the primary bicarbonate-generating base has been shown to worsen hyperlactatemia in patients who have severe circulatory instability with tissue hypoperfusion, and in patients with severe liver compromise. The 2012 KDIGO AKI guidelines suggest using bicarbonate-based solutions for all patients with AKI at a low level of evidence (2C), but recommend using such solutions more strongly for patients with liver
failure and/or lactic acidosis (level of evidence 2B) and for patients in circulatory shock (level of evidence 1B).
d.
Citrate-based solutions. These fluids evolved from attempts to merge the buffering and anticoagulation properties of citrate, and the need to simplify complex regional citrate anticoagulation (RCA) protocols. The bulk of citrate-based fluids have to be administered pre-filter to allow adequate filter anticoagulation. Forty to 60% of citrate infused in predilution mode is removed in the effluent, and the remainder is mainly metabolized by the liver into bicarbonate (1 mmol citrate yielding 3 mmol bicarbonate). Therefore, it is not appropriate to use these solutions in C-HD where dialysate flow is countercurrent to blood, or C-HF/HDF with predominantly postfilter replacement. Preparations containing citrate at concentrations of 11-12 mM may not provide adequate buffering capacity (
Naka, 2005). Fluids with a higher citrate concentration (14 mM) provide better acidosis correction with improved filter life (
Egi, 2005, 2008). Citrate-based solutions at 18 mM are available but the acid-base consequences have not been adequately studied. It is best to infuse citrate-containing replacement fluid predilution, adjusting the flow to achieve an optimum prefilter citrate to blood flow ratio, and then additional solute removal can be achieved by using bicarbonate-based solutions given either as dialysate or postdilution replacement fluid. Additional methods of citrate anticoagulation as well as potential advantages of this approach are discussed later in this chapter.
2.
Sodium. Commercially available CRRT fluids usually contain physiologic sodium concentrations at or close to 140 mM. When treating patients with severe, and especially longstanding, hyponatremia, where the goal is to slowly increase the serum sodium at a rate no greater than 6-8 mmol/L per day, the replacement fluid or dialysis solution needs to be diluted with water, to a concentrate just slightly greater than the predialysis sodium value. For details please see
Yessayan et al (2014). In some anticoagulation methods requiring infusion of trisodium citrate into the blood lines, custom-made, low-sodium (100 mM) dialysis/replacement solutions can be used to limit the occurrence of hypernatremia.
3. Potassium. CRRT fluids with no potassium are suitable for initial treatment of AKI patients with severe hyperkalemia. Once serum potassium has decreased to a safe level, fluids containing 4 mM potassium are used to minimize arrhythmia risk and depletion of body potassium. Commercially made fluids come premixed with potassium concentrations of 0, 2, or 4 mM. The lower potassium content solutions also may be used as needed in patients who are highly catabolic with persistent hyperkalemia.
4.
Phosphate. Hypophosphatemia during extended CRRT is common and can result in respiratory muscle weakness and prolonged respiratory failure in critically ill patients (Demirjian, 2011). Phosphate replacement for severe hypophosphatemia is routine, but frequent monitoring of serum phosphorus levels is necessary. Off-label addition of phosphate to CRRT fluid to maintain a level of 1.2 mM has been reported to maintain serum phosphorus with good clinical efficacy (
Troyanov, 2004). A replacement solution containing phosphate at 1.2 mM and bicarbonate at 30 mM is available, but its use has been associated with mild metabolic acidosis and hyperphosphatemia compared with conventional CRRT fluids (
Chua, 2012). The ideal fluid phosphate content should probably be lower, and further research is desirable.
AKI has been reported after phosphate enemas and after IV phosphorus infusion. In one audit, infusion of an IV solution of sodium/potassium phosphate containing 20 mM phosphate over an average of 5 hours was not associated with elevation of creatinine in patients with residual kidney function, but was associated with some reduction in ionized calcium concentration (Agarwal, 2014).
5. Calcium and magnesium. Most dialysis/replacement solutions contain 1.5-1.75 mM of calcium and 0.5-0.6 mM of magnesium, and their use usually allows maintenance of desired systemic levels. During RCA, citrate binds to and depletes serum calcium and magnesium. CRRT solutions used during RCA often contain no calcium to facilitate reduction of ionized calcium in the filter by citrate to allow adequate circuit anticoagulation. With RCA, separate systemic infusions of calcium and sometimes magnesium with strict monitoring protocols are thus necessary.
6.
Glucose. Modern CRRT solutions are either glucose free, or contain physiologic glucose concentrations, usually 5.5 mM (100 mg/dL). Use of glucose-free fluids in CRRT is associated with hypoglycemia, and glucose-containing CRRT fluids are preferred; regular monitoring and administration of insulin is necessary to prevent hyperglycemia and to achieve a target serum glucose of 6-8 mM, a level that has been associated with the best outcomes. Another argument against use of glucose-free CRRT solutions is that substantial amounts of glucose can be removed from the body with their use, and this may adversely affect nutritional balance (
Stevenson, 2013).
B.
Methods of preparing bicarbonate-based CRRT solutions when prepack-aged solutions are not available. Customized solutions can be prepared in the pharmacy, or by the dialysis machine in the form of ultrapure solutions; the latter is appropriate only in countries where regulatory approval for online hemodiafiltration
has been granted. One can prepare sterile dialysis/replacement fluid manually to achieve solutions containing 30-35 mM bicarbonate. Bicarbonate is in equilibrium with carbonic acid, which breaks down to CO2 and H2O; therefore, bicarbonate solutions are unstable. Bicarbonate also forms insoluble salts when in solution with calcium and magnesium. Therefore, bicarbonate-based dialysis/replacement solutions should be prepared just before use.
1. Single-bag method. Dialysis or replacement solution containing bicarbonate and no lactate is made by adding (usually this is done by the hospital pharmacy service) NaHCO3 and some additional NaCl to 0.45% NaCl obtained commercially. A small amount of CaCl2·2H2O is added as well, and magnesium is given parenterally as needed.
Formulation: 1.0 L of 0.45% NaCl + 35 mL of 8.4% NaHCO3 (35 mmol) + 10 mL of 23% NaCl (40 mmol) + 2.1 mL of 10% CaCl2·2H2O (1.45 mmol or 2.9 mEq); total volume = 1.047 L.
Final concentrations in mM: Na, 145; Cl, 114; HCO3, 33; and Ca, 1.35 (2.7 mEq/L).
2. Two-bag method. Bags of 0.9% saline with added calcium are alternated with bags of 0.45% saline with added bicarbonate.
Formulation: Solution A: 1.0 L of 0.9% saline + 4.1 mL of 10% CaCl2·2H2O (2.8 mmol or 5.6 mEq). Solution B: 1.0 L of 0.45% saline + 75 mL of 8.4% NaHCO3 (75 mmol); total volume = 2.079 L.
Final concentrations in mM (when considered together): Na, 147; Cl, 114; HCO3, 36; and Ca, 1.35 (2.7 mEq/L).
3.
Dialysis machine method (C-HD only). One can prepare bicarbonate-containing dialysis solution for C-HD by ultrafiltering dialysis solution prepared by a standard dialysis machine across a dialyzer (to remove bacteria) and storing the solution in a 15-L sterile drainage bag from a peritoneal dialysis cycler. Such solutions should be used promptly after preparation. This technique has since been modified by storing solutions in more convenient 6-L sterile bags. The fluid preparation does not show growth of bacteria for at least 72 hours and for up to 1 month in tests. Routinely, however, the bags are discarded if not used within 72 hours of preparation by protocol. In 10 years of use, there have been no reported adverse events, and Limulus amebocyte lysate assays for endotoxin are reliably below the limit of detection (
Teo, 2006).
C. Sterility. Sterile dialysis solution is used for C-HD and C-HDF because the slow dialysate transit time plus the extended time of use of the same circuit and dialyzer could otherwise encourage bacterial growth in the dialysate circuit. All replacement fluid infusions given directly into the blood lines must be sterile.