Urinary symptoms related to multiple sclerosis (MS) present a complex challenge for the treating physician. However, several treatment options are available for the symptomatic patient once the physician understands basic MS disease epidemiology and pathophysiology. Depending of disease status and symptoms, MS urinary symptoms may respond to directed behavioral, pharmacologic, intravesical, neuromodulation, or surgical therapies.
Epidemiology of multiple sclerosis
Multiple sclerosis (MS) is the most common neuroinflammatory disease of the central nervous system (CNS), and is characterized by plaques of demyelination in CNS white matter. The disease affects approximately 85 cases per 100,000 people and is more prevalent in northern than southern latitudes. The initial onset of neurologic symptoms typically occurs between ages 20 and 50 years, and presentation at an age older than 40 may be associated with a greater risk of progressive disability. Women are 2 to 4 times more commonly affected than men. Although researchers have cited the Epstein-Barr virus, ultraviolet radiation, tobacco, vitamin D deficiency, and smoked meat with nitrites as potential environmental risk factors for developing MS, familial clustering and twin studies suggest risk is more related to a complex interaction between genetically susceptible patients and their specific environments.
Classification
Clinically, MS presents as acute neurologic compromise, and most patients cite symptoms of numbness, decreased of motor strength, and loss of coordination and/or vision. MS can be diagnosed by the McDonald Criteria, which require magnetic resonance imaging (MRI) findings to correlate with 2 neurologic clinical manifestations, separated by time and recovery. A spinal tap showing oligodonal bands also supports the diagnosis when no recovery has occurred. Disability is commonly reported in the literature through the Extended Disability Symptom Score (EDSS), which measures disease impact on pyramidal (voluntary), brainstem, visual, cerebral (memory), cerebellar, sensory, and bowel/bladder systems. A single score is given, which ranges from 1 (minimal impact) to 10 (death from disease). Patients with scores higher than 6.5 require constant bilateral assistance to walk 20 m without resting and have impact on 2 systems. The severity of urinary symptoms appears to be associated with EDSS scores and usually correlates with pyramidal symptom severity.
MS can be roughly categorized into 3 different disease progressions :
Relapsing/Remitting : Eighty percent of MS patients will experience symptoms followed by complete or partial resolution after 2 days to 6 weeks. There is no progression of disease between episodes. The initial relapse rate is approximately 0.3 episodes per year, but frequency decreases as time progresses.
Secondary Progressive : Fifty percent of relapsing/remitting patients will develop progressive neurologic decline, particularly in the lower extremities. Secondary Progressive MS typically occurs more than 10 years after initial diagnosis and is characterized by less recovery from symptomatic episodes.
Primary Progressive : Ten percent of patients will experience continuous neurologic degradation after initial presentation, characterized by no remission.
Classification
Clinically, MS presents as acute neurologic compromise, and most patients cite symptoms of numbness, decreased of motor strength, and loss of coordination and/or vision. MS can be diagnosed by the McDonald Criteria, which require magnetic resonance imaging (MRI) findings to correlate with 2 neurologic clinical manifestations, separated by time and recovery. A spinal tap showing oligodonal bands also supports the diagnosis when no recovery has occurred. Disability is commonly reported in the literature through the Extended Disability Symptom Score (EDSS), which measures disease impact on pyramidal (voluntary), brainstem, visual, cerebral (memory), cerebellar, sensory, and bowel/bladder systems. A single score is given, which ranges from 1 (minimal impact) to 10 (death from disease). Patients with scores higher than 6.5 require constant bilateral assistance to walk 20 m without resting and have impact on 2 systems. The severity of urinary symptoms appears to be associated with EDSS scores and usually correlates with pyramidal symptom severity.
MS can be roughly categorized into 3 different disease progressions :
Relapsing/Remitting : Eighty percent of MS patients will experience symptoms followed by complete or partial resolution after 2 days to 6 weeks. There is no progression of disease between episodes. The initial relapse rate is approximately 0.3 episodes per year, but frequency decreases as time progresses.
Secondary Progressive : Fifty percent of relapsing/remitting patients will develop progressive neurologic decline, particularly in the lower extremities. Secondary Progressive MS typically occurs more than 10 years after initial diagnosis and is characterized by less recovery from symptomatic episodes.
Primary Progressive : Ten percent of patients will experience continuous neurologic degradation after initial presentation, characterized by no remission.
MS and bladder physiology
Impact of Lesion Location
Imaging and anatomic studies suggest that patients with MS lesions located in specific CNS regions are more likely to display prominent urinary symptoms. In general, lesion volume modestly correlates with symptom progression within the first 5 years. Charil and colleagues studied 452 MRI scans from relapsing and remitting MS patients, and found patients with lesions in the medial frontal lobes, cerebellum, insula, dorsal midbrain, and pons scored significantly lower on urinary-specific quality of life assessments. Likewise, other studies have found a strong association on MRI between midbrain lesions and loss of urinary control. Patients with cerebellar or specific frontal lobe lesions may display urinary incontinence or urinary retention due to loss of cognitive function or awareness of bladder volumes. Spinal plaques affecting the corticospinal tracts are also common, and are responsible for symptom progression measured by the EDSS. Up to 80% of MS patients will have cervical spinal cord involvement and are likely to display some urinary hesitancy and retention.
With the exception of detrusor-external dyssynergia (DSD), there is conflicting evidence regarding lesion location and specific, reproducible urodynamic findings. Kim evaluated 90 patients with symptomatic MS (EDSS scores not reported) and found no clear relationship between urodynamic findings, MRI lesion location, International Prostate Symptom Scores, and lesion size. Other studies reported some associations between high-volume cortical lesions and detrusor overactivity, pontine lesions and detrusor areflexia, and pontine to conus medullaris lesions and ice water–induced detrusor contractions. Sacral cord lesions are less common in MS but can be associated with detrusor hypocontractility and atony on urodynamic study. Detrusor-sphincter dyssynergia appears to have a strong correlation with MS lesions in the cervical spinal cord. A strong relationship between DSD and cervical spinal cord lesions or injuries has also been described in spinal cord injury pathologies.
Impact of Disease Process
MS likely causes clinical symptoms, including urinary symptoms, through demyelization and axonal degradation. Demyelination is thought to develop before degradation. Using experimental autoimmune encephalomyelitis animal models, research suggests that CD4+ and CD8+ T cells along with macrophages induce and then maintain an inflammatory reaction against the axonal myelin sheath, which ultimately results in demyelination. Autoimmune antibodies from B calls may also play a role in maintaining inflammation along the axon. Axonal degradation may occur over time as the axon becomes more vulnerable to stress and degrades due to inhibition of remyelination, exposure of an increased number of Na + channels, and decreased adenosine triphosphate production in the demyelinated area. Although there is a clear relationship between plaque development and clinical symptoms, axonal degradation may be more strongly associated with progression of symptoms than previously appreciated.
MS CNS lesions may also exert a local effect on the bladder function. It is known that any CNS lesion can cause previously silent unmyelinated C fibers in the bladder to become increasingly mechanosensitive to detrusor wall stretching. Radziszewski and colleagues performed urodynamics and trigonal bladder biopsies from 18 symptomatic MS patients, and found a greater density of substance P and calcitonin gene related peptide–sensitive unmyelinated C fibers in patients with detrusor overactivity. Findings such as these suggest that biopsies measuring C-fiber neuronal density may be used in the future to gauge a patient’s potential response to intravesical treatments. Other changes in bladder structure may also occur in the setting of MS, such as alterations in the ultrastructure of the bladder lamina propria and increased number of Schwann cells. The clinical impact of these findings is unclear, and more research is needed to correlate pathology with clinical symptoms.
Presentation of Urinary Symptoms
In the North American Research Committee on Multiple Sclerosis (NARCOMS) cross-sectional survey of 9700 MS patients, 65% reported at least moderate to severe urinary symptoms. Urinary urgency is usually the most prevalent reported symptom for MS patients. Compared with an unaffected population in a cross-sectional study of 12,570 Canadian women, patients with multiple sclerosis were 7.6 times more likely to report bother from overactive bladder symptoms. In some studies, up to 21% to 50% of patients experience frequent episodes of urinary incontinence in addition to hesitancy and 25% of patients report frank urinary retention. Phadke’s review of MS epidemiology suggests that 0% to 14% of MS patients will report urinary complaints as the initial MS symptom and mean time to onset of symptomatic urinary dysfunction for most MS patient ranges between 6 and 8 years after initial diagnosis. However, changes in urinary symptoms have been noted as early as 2 years after initial diagnosis are usually associated with an increasing postvoid residual (PVR) and a worse disease course.
Clinical evaluations
Urodynamics
Urinary symptom type does not always predict urodynamic findings because different voiding dysfunction pathologies can present with the same common urinary complaint. In a prospective study of 212 MS patients by Koldewijn and colleagues, 27% complained of obstructive/retention urinary symptoms. When examined with urodynamic tests, 8% of the sample had detrusor hypoactivity, 13% had DSD, and 5% had other unspecified bladder outlet obstruction. Other investigators have also commented on the low correlation between urinary symptoms and urodynamic findings for MS patients. Furthermore, 70% of MS patients presented with combined symptoms of obstruction and incontinence in one study, suggesting that multiple voiding dysfunction patterns can coexist.
MS patients at the author’s institution undergo urodynamic testing for symptoms of refractory urinary incontinence, obstruction/hesitancy, urinary retention, and elevated PVR greater than 150. All urodynamic studies for MS patients should use standardized approved urodynamics practice recommendations from the International Continence Society. Electromyography and/or fluoroscopy are needed to diagnose DSD during the studies, but there is no consensus on the best technique for identifying this pathology. The author relies on electromyography (EMG) to assess DSD and has found fluoroscopy to be helpful in identifying DSD, bladder diverticuli, and vesicoureteral reflux.
There are multiple small descriptive studies reporting on urodynamic findings in MS patients. Litwiller and colleagues performed a meta-analysis on urodynamic data from 22 studies, comprising of 1882 MS patients, and found detrusor overactivity (then called hyperreflexia) in 62% of the total sample, DSD in 25%, hyporeflexia in 20%, and no findings in 10%. There are data suggesting that MS patients with neurogenic detrusor overactivity will demonstrate greater amplitude of detrusor contraction compared with idiopathic detrusor overactivity. Of note, MS women with DSD did not display higher voiding pressures in a subsequent study. New urodynamic findings can present or old findings change as the disease progresses. Ciancio and colleagues followed 14 MS patients over a mean 42-month follow-up, and found 5 patients with lower bladder compliance and 6 with new voiding pathologies. Given the multiple and changing urodynamic findings in MS patients, the author believes that patients can benefit from serial urodynamic testing as the disease progresses.
Urinary Tract Infections
Urinary tract infections (UTI) are also prevalent in the MS population, and patients with DSD may be at particular risk for pyelonephritis. Evidence suggests that systemic bacteria infections may exacerbate the autoimmune response in MS. The frequency at which an MS patient experiences UTI may also increase the risk of symptom progression. Risk factors for UTI have not been completely identified, although an elevated PVR and DSD may be potential variables. More research is needed to identify other potential risk factors unique to the MS population. Expert opinion suggests that all MS patients with symptomatic, recurrent UTIs be evaluated with a PVR and upper tract imaging studies. At the author’s institution, urodynamics is also performed to evaluate dysfunction voiding physiology as a risk factor.
Upper Tract Changes
There is considerable controversy surrounding MS and risk of upper urinary tract degeneration. The reported prevalence of upper tract changes in the MS population has ranged widely from 0.9% to 17%. de Sèze and colleagues compiled data from 11 studies consisting of 1200 MS patients and found a rising incidence of upper tract complications over time for patients symptomatic with MS. These data suggested that upper tract changes were most likely to occur after 6 to 8 years with disease. Time with disease, increased patient age, and pyramidal symptoms have also been associated with an increased risk for upper tract changes in some studies, but others do not associate upper tract changes with any risk factors. Care should be applied when extrapolating data from these studies because patients in each cohort could represent different patient populations, access to care, and different MS treatment regimens.
Cancer
Bahmanyar and colleagues assessed cancer risk in 20,276 MS patients over 35 years of follow-up and found an increased risk for developing urinary organ cancer, excluding kidney, prostate (hazard ratio 1.27, 95% confidence interval 1.05–1.53). The cause of this is unclear, but it may represent surveillance bias. In addition, MS patients treated with cyclophosphamide may be at increased risk for bladder cancer.
Treatment
In a disease marked by relatively poor control of symptoms, urinary complaints are a point of intervention that can be addressed and improved for a large number of MS patients. Maintaining adequate urinary control may also have additional benefits on sexual health and mental health, particularly in MS patients with increasing disability. Consequently, a plan of increasing intervention can be employed to address both safety- and urinary-specific quality of life for these complex patients. The treatment plan used at the author’s institution is presented in Fig. 1 . Additional algorithms have also been published.
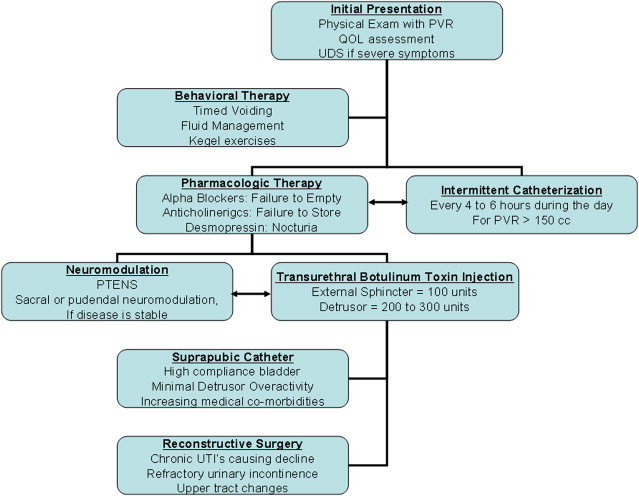
Behavioral Therapy
Behavioral therapy, including interventions such as pelvic floor muscle training (PFMT) and fluid management, has been an effective treatment strategy for women with idiopathic overactive bladder symptoms. Because many MS patients have pelvic floor muscle spasms in addition to voiding dysfunction, PFMT therapies, in theory, can yield some symptomatic improvements. However, there are few studies examining the efficacy of behavioral therapy for neurogenic detrusor overactivity related to MS. De Ridder and colleagues demonstrated improvement in mean functional bladder capacity (178 mL to 205 mL) and reduction in mean number of urinary frequency episodes (12.7 to 9.1) after 1 month of PFMT. Combining behavioral therapy modalities may also offer benefit for MS patients. McClurg and colleagues randomized 30 women with MS into treatment groups of PFMT, PFMT + EMG biofeedback, and PFMT + EMG biofeedback + neuromuscular stimulation, and found improved efficacy in reducing the number of leaking episodes ( P = .014) and in volume leaked ( P = .0001) when all 3 modalities were used compared with PFMT alone or PFMT + EMG. A larger double-blinded study by the same group noted an 85% reduction of urinary incontinence episodes with neuromuscular stimulation + EMG + PFMT, compared with 47% reduction in the PFMT + EMG + placebo group.
Pharmacologic Therapy
Oral pharmacologic therapy in the MS population targets 3 prevalent urinary symptoms: urgency/urge incontinence, urinary retention, and nocturia.
Anticholinergics
Although anticholinergic medications are recommend to treat urge/urge incontinence in multiple sclerosis, there are few studies examining efficacy. Fader and colleagues compared intravesical atropine to oxybutynin immediate release and found a small difference in posttreatment bladder capacity (55 mL improvement with oxybutynin vs 79 mL with atropine) but no difference in incidence of incontinence events. Other studies have also demonstrated symptom improvement with increasing anticholinergic medication dosage, or using anticholinergics to convert detrusor overactivity to detrusor hypocontractility. However, Nicholas and colleagues performed an analysis on all current literature regarding anticholinergic therapy for MS-related incontinence and found only 5 usable studies, none of which included placebos or long-acting anticholinergic medications. No conclusions suggesting benefit from anticholinergic usage could be drawn from the analysis. In addition to the lack of outcome studies, physicians are likely undertreating urgency/urge incontinence in the MS population. According to the NARCOM survey, only 43% of patients with significant urinary urgency had seen a urologist and only 51% were treated with an anticholinergic. Alternative pharmacologic agents, such as cannabis, have demonstrated some efficacy in reducing MS-related urinary incontinence, but use of this medication is only investigational at this point in time.
α-Blockers
There is also little information regarding pharmacologic treatment of MS-related urinary retention. O’Riordan and colleagues noted a 41% improvement in flow rate and a 26% reduction of PVR with MS patients treated with an α-blocker for 4 weeks. Stankovich and colleagues noted a 54% decrease in International Prostate Symptom Score for 28 MS patients (20 female) with DSD who were treated with tamsulosin, 0.4 mg for 2 months. Additional studies of mixed DSD pathologies, including MS, also found tamsulosin to be effective in treating obstructive symptoms.
Desmopressin
By contrast, there are many studies investigating the efficacy of desmopressin, 20 μg, for MS-related nocturia. While on desmopressin, MS patients experienced a decrease in nocturia by mean 0.5 to 1.5 episodes per night and an increase of uninterrupted sleep by mean 2 hours. A high bladder capacity and compliance may predict better outcomes for desmopressin in MS patients. Reduction in urinary frequency is limited to the first 6 to 8 hours after delivery. Urine sodium and maximal urinary daily output remained relatively stable in a meta-analysis of MS patients on desmopressin.
Catheterization
MS patients may require some assistance in bladder emptying because of DSD, detrusor atony, and/or reduced physical mobility. In the NARCOMS survey, 37% of MS patients reported using catheter assistance for bladder emptying. Catheter usage correlated with advance disease. Although the PVR volume which places patients at most risk for urinary pathologies is not known, the United Kingdom consensus on management for multiple sclerosis urinary symptoms recommends teaching clean intermittent catheterization (CIC) to patients with PVRs greater than 100 mL. In most CIC treatment plans, patients catheterize according to a set time schedule, ranging from every 3 to 6 hours. CIC can be easily taught to most MS patients, and at least one study reported successful CIC education for 87% of MS patients. However, more instruction was needed for patients with cognitive decline and higher EDSS. Fakas and colleagues examined the risk of UTIs for MS patients on CIC, and found that while 90% of these patients developed bacteriuria, only 14% developed symptomatic urinary tract infections. In general, CIC seems to be a safe, well-tolerated management strategy for the symptomatic MS patient.
However, controversy surrounds the use of indwelling urinary catheters for refractory MS urinary symptoms. Although studies have demonstrated severe urethral damage and erosion from long-term urethral catheterization in MS patients, the true incidence of urethral and bladder complications from urethral catheters is not known for the MS population. If an indwelling catheter is indicated, the United Kingdom consensus statement recommends offering a suprapubic instead of a urethral catheter. Although commonly used, long-term complications of suprapubic catheterization have not been reported for the MS population regarding patient satisfaction, rate of UTI, loss of bladder compliance, and risk of bladder cancer.
Intravesical Agents/Botulinum Toxin
Intravesical agents have been used to treat MS-related urinary urgency and incontinence. Several trials of mixed multiple sclerosis and spinal cord injury patients have found intravesical installations of capsaicin or resiniferatoxin superior to placebo in reducing neurogenic urinary urgency/frequency. When comparing these vanilloid agents, resiniferatoxin treatments resulted in fewer incontinent episodes per day (60% improvement with capsaicin, 94% improvement with resiniferatoxin) and capsaicin treatments were associated with more short-term pubic pain during installation. However, a meta-analysis did not yield a consistent treatment effect for vanilloid agents. Given the questionable efficacy of anticholinerigics in treating MS-related urgency/incontinence, more research is needed to develop an efficacious, easy to use, intravesical agent for the symptomatic MS patient.
Botulinum toxin A injections represent a significant advancement in treatment options for neurogenic voiding dysfunction. Although there are no evidence-based guidelines suggesting optimal injection location or dose, 10 units/mL injections usually are spaced across the posterior detrusor and the trigone is spared to minimize the risk of vesicoureteral reflux. There are multiple trials that suggest intradetrusor botulinum toxin injections can improve cystometric capacity by 50% to 300%, improve time to first desire by 50%, and reduce the number of incontinence episodes by 40% to 77%. Botulinum toxin has also been used to decrease urethral leakage in the setting of an indwelling urinary catheter. In contrast to the spinal cord injury literature, botulinum toxin injections into the external sphincter did not demonstrate improvement in PVR in a recent placebo-controlled trial, but smaller observational trials have suggested efficacy ( Table 1 ). In general, toxin efficacy is limited in duration, and most studies suggest benefits begin to wane after 6 months. Botulinum toxin–specific a ntibodies have also been detected after injections and may be a source of treatment failure over time. At present, botulinum toxin is not approved by the US Food and Drug Administration for use in the bladder. Consequently, treating physicians should obtain detailed informed consent from patients and follow institution guidelines for off-label medication usage.








