Fig. 29.1
Deep pelvic dissection anatomy
Bleeding
Hemorrhage can occur suddenly when the presacral fascia is breached and pelvic veins are injured. Manual pressure or suture ligation can curtail most presacral venous bleeding. Hemoclips, packing, electrocautery, and topical agents such as absorbable gelatin sponges (Gelfoam), microfibrillar collagen (Avitene), and oxidized regenerated cellulose (Surgicel) can be used for hemostatic purposes for mild presacral bleeding. Conversely, injury to the basivertebral veins can cause massive, life-threatening bleeding if not recognized early and appropriately managed. Hemorrhage from these veins cannot be controlled with suture ligature, cautery, or topical hemostatic agents. The basivertebral veins are bridging veins between the internal vertebral venous system which lies deep to the sacrum and the anterior external venous plexus which resides on the anterior surface of the sacrum between S3 and S5. These veins are most commonly injured when blunt dissection is used for the posterior dissection. If the site of bleeding can be identified, pressure from a single finger should be able to adequately temporize the bleeding while resources are mobilized. A titanium thumbtack advanced through the bleeding point into the sacrum can be effective in controlling massive bleeding. Other authors have advocated “welding” a strip of skeletal muscle into the sacral venous orifice using high current electrocautery. Any attempts at definitively stopping this type of bleeding should be done when the patient is adequately stabilized and appropriate blood products are available for resuscitation. When bleeding is uncontrollable, the pelvis should be packed for 24–48 h with subsequent return to the operating room to complete the procedure.
Surgical Site Infection
Estimated to occur in up to 30 % of resections. surgical site infection (SSI) is the greatest contributor to surgical morbidity after rectal cancer surgery. Risk factors for SSI include immunosuppression, malnutrition, diabetes mellitus, prior irradiation, fecal contamination during surgery, extensive surgery, length of hospitalization prior to surgery, age >60, and ASA class >2 [1, 26–29]. SSI is divided into superficial incisional, deep incisional, and organ/space infections.
Superficial incisional infection is characterized by localized swelling, erythema, warmth and purulent drainage and is initially treated by opening the wound, followed by routine dressing changes. Antibiotics should be initiated if cellulitis persists despite wound decompression. Deep incisional infection involves the rectus muscle and/or the fascia and may result in fascial dehiscence. Treatment includes IV antibiotics, wound exploration with debridement of infected tissues, and repair of fascial dehiscence.
Necrotizing fasciitis (NF) is a rare, but potentially fatal deep incisional infection caused by toxin-producing, virulent bacteria such as group A hemolytic Streptococcus and Staphylococcus aureus [30]. Characterized by rapid and extensive soft tissue and fascial necrosis, unrecognized NF can swiftly lead to widespread organ failure and death. After the initial innoculation, an opportunistic polymicrobial infection of aerobic Gram negative and anaerobic organisms ensues in this hypoxic environment. Early diagnosis accompanied by aggressive multi-disciplinary intervention is essential. Presenting symptoms of NF include a disproportionate amount of pain and tenderness around a wound with minimal skin changes, quickly followed by fever, erythema, crepitus, skin mottling, blistering and sloughing. Resuscitation including broad-spectrum antibiotics (e.g. Penicillin G, Clindamycin, and Gentamicin), followed by aggressive operative debridement of all necrotic and infected tissues is mandatory for preventing death from septic shock.
Typically presenting within the first postoperative week, most organ/space infections are precipitated by intraoperative fecal contamination, unrecognized enterotomies, or anastomotic leaks. Clinical symptoms such as fever, leukocytosis, abdominal and/or pelvic pain should prompt the ordering of a CT scan of the abdomen and pelvis to investigate for intraabdominal or intrapelvic abscess. Rectal contrast can help identify a potential fistulous connection from the anastomosis to a nearby collection. First-line treatment should consist of broad spectrum antibiotics and percutaneous drainage of abscesses greater than 2 cm. Reimaging, including administration of contrast through the drain, should be performed after several days of bulb suction drainage to check for persistence of fluid. Recalcitrant pelvic sepsis may warranted operative management, including proximal fecal diversion.
Perineal wound infection, a form of deep incisional infection, occurs in up to 50 % of abdominoperineal resections. The circumferential excision of the distal rectum and anal canal through the pelvic floor results in a poorly perfused dead space that is susceptible to bacterial overgrowth. When a wide resection through the pelvic floor is performed (either due to a bulky or locally invasive tumor or to surgeon preference for an extralevator perineal resection (ELAPE)), there may be very little levator muscle available to close the perineal defect. Ischiorectal fat is left for reapproximation, creating a closure with a notoriously poor blood supply. A closed space infection may ensue that can present with perineal pain, foul-smelling drainage, and wound dehiscence. The incision should be opened and locally explored for fluid collections. Broad-spectrum antibiotics with gram negative and anaerobic coverage should be instituted. Wound care consisting of either gauze packing or negative pressure therapy (VAC) can help expedite healing by secondary intention. Interestingly, wound dehiscence, after APR, regardless of infection, has been associated with decreased survival [31].
Clostridium Difficile
Clostridium difficile-associated colitis is caused by toxins secreted by the eponymous Gram positive anaerobe and is associated with symptoms ranging from mild, watery diarrhea to fulminant colitis. Reports of C. difficile colitis after colorectal resection range from 1.3 to 21 % with a greater predilection in the patient with immune system dysfunction, fecal stasis, long-term antibiotic use, and chemotherapy administration [32–34]. First-line treatment includes stopping antibiotics and initiating either metronidazole or vancomycin. Surgical intervention may be necessary if the patient does not respond to treatment or clinical course worsens. The mortality rate for patients who progress to fulminant colitis or toxic megacolon is 35–80 % [32].
Thromboembolism
Venous thromboembolism (VTE) frequently occurs in the setting of malignancy and represents a spectrum of diseases including deep vein thrombosis (DVT) and pulmonary embolus (PE). Specific to rectal cancer treatment, risk factors for VTE include tumor factor activation, intravascular inflammation resulting from chemotherapy and radiation therapy, extensive abdominal and pelvic surgery, and prolonged immobilization. Prophylactic anticoagulation should be initiated prior to surgery and continued for at least 7–10 days. High-risk patients should extend the prophylaxis for a total of 4 weeks. Weight-based low-weight molecular heparin (LWMH) is recommended for extended prophylaxis.
Deep vein thrombosis is typically heralded by unilateral extremity swelling, warmth, and erythema. A palpable cord may be appreciated. Although lower extremity DVTs occur more frequently, upper extremity DVTs may also be seen, especially in the setting of an indwelling central venous catheter. Duplex venous ultrasonography is the recommended diagnostic modality. Initial treatment includes subcutaneous LMWH, IV unfractionated heparin (UFH), monitored subcutaneous UFH, or subcutaneous fondaparinux. Acute DVT should be treated for at least 5 days and until the INR is ≥2.0 for 24 h. A vitamin K antagonist (VKA) (e.g. warfarin) should be initiated with LMWH, UFH, or fondaparinux on the first treatment day. VKA should be adjusted to maintain a target INR of 2.5 for 3–6 months. An inferior vena cava (IVC) filter can be placed in patients who are high-risk for bleeding, but anticoagulation should subsequently be initiated if the bleeding risk resolves.
Pulmonary embolus is a dreaded complication that occurs when a DVT propagates through the venous system into the pulmonary vessels. Symptoms may include dyspnea, tachnypnea and pleuritic chest pain. Acute cardiopulmonary collapse may ensue. Treatment includes hemodynamic stabilizatiion, systemic thrombolytic therapy, and subsequent anticoagulation for 3–6 months. Routine IVC filter placement is not recommended.
Indefinite anticoagulation is advised for both DVT and PE in patients with a thrombotic diathesis and with metastatic malignancy.
Anastomotic Issues
Anastomotic complications can be devastating after restorative rectal cancer surgery. Bleeding, leak, and stricture are the most commonly encountered complications after low anterior resection.
Anastomotic Bleeding
Minor bleeding or oozing from the anastomotic staple line is common and can be easily managed with manual pressure, interrupted suture ligation of a discrete portion of the anastomosis, or circumferential suture reinforcement of the entire staple line. Cautery can be used sparingly for hemostasis, but caution should be exercised to prevent a thermal burn that could then lead to a delayed anastomotic disruption.
Postoperative bleeding typically presents as the passage of bloody stools or clots after restitution of bowel function. Conservative management, including assessment of vital signs, serial hemoglobins, and fluid resuscitation, should be initially employed. Transfusion of packed red cells may be needed if the blood loss is substantial. Coagulopathies should be corrected with blood products, factors, and/or vitamin K.
Persistent bleeding and massive hematochezia, despite conservative efforts, are rare, but warrant more aggressive management. After proper resuscitation, proctoscopy should be performed. Care must be taken not to disrupt the fresh anastomosis while adequately investigating the nature of the bleed and suctioning out intraluminal contents. Proctoscopy with lavage and evacuation of the clot may suffice to curtail the persistent oozing. A 1:100,000 saline solution with epinephrine can be instilled into the rectum and left for 5–10 min prior to reevaluation. Oversewing of the low colorectal anastomosis can also be performed. For the more proximal anastomosis, flexible endoscopic evaluation may be warranted in which hemoclips can be deployed and epinephrine can be injected into the bleeding site.
Anastomotic Leak
Anastomotic leak (AL) is one of the most dreaded complications of rectal cancer surgery. Subdivided into clinically recognized (clinical leak) and unrecognized (subclinical leak) anastomotic disruptions, AL can present either early in the postoperative period or in a delayed fashion (>30 days after surgery). The incidence of clinical leak ranges from 2 to 36 % with an associated mortality rate between 6 and 22 % [27, 35–37] AL is associated with decreased health-related quality of life (HRQL), especially when poor functional results, anastomotic stricture, or diverting ostomy are involved [35, 37] Signs and symptoms of AL include fever, sepsis, and peritonitis in a patient with radiologic evidence of free extravasation of intraluminal contrast, a contained perianastomotic fluid collection, or a presacral fluid collection. Succus draining to the skin, vagina, or urethra may also be a harbinger of an AL complicated by fistula.
Patient factors, such as male sex, poor nutritional status, compromised immune system, history of prior pelvic radiation, and comorbidities such as morbid obesity and diabetes mellitus, have been associated with an increased risk for AL [35, 37, 38].
There appears to be inverse relationship between AL and the location of the tumor, as distal lesions notoriously present greater surgical difficulty. Vignali et al. reported a 7.7 % AL rate with low anastomoses (<7 cm from the anal verge), while only a 1 % AL rate with high anastomoses (>7 cm from the anal verge) [39]. Resection of distal tumors may also lead to the truncation of oncologically safe distal margins when sphincter preservation is attempted. Cong and colleagues found in a multivariate analysis that AL was 6.18 times greater in patients with distal margins <1 cm compared to those with distal margins ≥1 cm (p = 0.009) [40].
Interestingly, AL rates surged after the introduction of the TME technique. These early studies reported clinical leak rates ranging from 16 to 23 % [27, 41–43]. It had been suggested that TME endangered the blood supply of the rectal stump, however increased experience with TME, including laparoscopic TME, has resulted in comparable AL rates to non-TME procedures [38, 44–51].
While AL is not prevented by fecal diversion, the severity of the clinical presentation of AL has been lessened by the presence of a proximal loop ileostomy [11, 52].
While a CT scan of the abdomen and pelvis may demonstrate free intraabdominal air and fluid, a gastrografin enema can be more useful in delineating the location and magnitude of a suspected AL. Luckily, the large majority of contained AL will heal with conservative management including systemic antibiotics and bowel rest. Ultrasound or CT-guided drain placement is recommended for walled-off fluid collections ≥2 cm.
Clearly, surgical intervention is warranted for the patient with free extravasation of contrast on CT scan, as well as, the unstable patient with clinical symptoms of intraabdominal sepsis. Fluid resuscitation and broad-spectrum antibiotic administration should be initiated early, followed by surgical exploration with liberal irrigation of any purulent and/or feculent ascites. Anastomotic integrity determines whether a primary repair can be attempted with or without proximal diversion. A takedown of the colorectal anastomosis with diverting end colostomy and closure of the rectal stump is recommended with a significant dehiscence, in the setting of substantial pelvic contamination, or in the unstable, septic patient. Pelvic drainage can be considered after primary repair of an anastomotic defect or when the rectal stump cannot be adequately closed.
Several technical principles should be adhered to in order to minimize AL. First, adequate blood supply to the two anastomotic stumps should be verified. Second, suturing or stapling of the anastomosis should be meticulously performed. This should be followed by a “leak test” in the operating room which involves insufflating the rectum with occlusion of the proximal bowel lumen, while submerging the anastomosis under saline or water. In addition, the integrity of the anastomotic rings should be carefully inspected. Lastly, a tension-free anastomosis should be created, which may warrant the release of the splenic flexure. High ligation of the inferior mesenteric artery and vein can provide additional laxity of the proximal bowel.
Anastomotic Stricture
Stricture is typically the long-term result of anastomotic ischemia, dehiscence, or leak. Disruption of more than 25 % of the luminal circumference will often result in excessive fibrosis of the anastomotic line. Stenosis can also be seen in the diverted patient as the anastomosis does not experience auto-dilatation from the passage of stool. Symptoms of stricture depend on the location of the anastomosis, the degree of stricture, and the consistency of stool. Patients may experience diarrhea, constipation, urgency, soiling, and tenesmus. As tumor recurrence can be heralded by such pronounced changes in bowel habits, endoscopic evaluation is important to differentiate between benign and malignant stricture. Benign strictures can be initially treated with stool softeners and enemas. Digital dilatation can be performed for low anastomoses while higher anastomoses (>7 cm) may require dilatation with Hegar’s dilators or balloon dilatation with a flexible endoscope. Refractory strictures may require either transanal incision of fibrotic scar or revisional surgery.
Incisional and Parastomal Hernia
The incidence of incisional and parastomal hernia after colorectal surgery is estimated to be up to 30 and 60 %, respectively [53–55]. Surgical site infection and morbid obesity are the two largest contributors to hernia formation [29, 54, 55]. Murray found patients who developed a SSI were more than 1.9 times as likely to develop an incisional hernia compared to those who did not have a SSI [29]. Schreinemacher [54] and DeRaet [56] reported significantly higher rates of incisional and parastomal hernias in patients with BMIs ≥30 and with waist circumferences in excess of 100 cm (Fig. 29.2). Other risk factors include low albumin levels, diabetes mellitus, chronic immunosuppression, male gender, anemia, and old age [55, 56]. Persistent coughing, abdominal distention, retching/vomiting, and development of excessive intraabdominal ascites in the postoperative period can contribute to tension on the fascial closure and should be minimized, if possible.
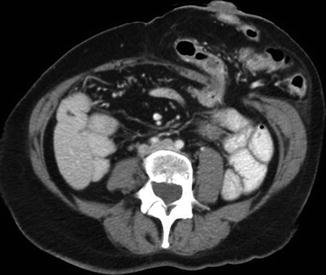

Fig. 29.2
Parastomal hernia (colostomy)
While multiple sources have reported decreased incisional hernia rates with laparoscopic resection for colorectal cancer compared to open surgery [57], increased hernia rates have been reported with laparoscopy at the specimen extraction site (especially with midline extraction) [58–61]. The data remain inconclusive regarding the potential protective effect of laparoscopy in regards to hernia formation [57, 62].
Perineal Hernia
Perineal hernia is a rare complication that can result after infection or poor healing of the perineal wound. Small bowel can bulge inferiorly, leading to skin breakdown and even evisceration. Symptoms range from pressure, pain, or fullness in the perineum to obstructive symptoms. Intraoperative attempts to prevent this complication include pelvic drain placement, closure of the pelvic parietal peritoneum, myocutaneous flap construction (gracilis, rectus abdominus, inferior gluteal), posterior rotation of the uterus, and placement of biologic mesh to bridge the pelvic hollow. Repair may also include flap construction or biologic mesh placement, necessitating both an anterior and posterior approach.
Ostomy Issues
Ostomy creation is fraught with potential complications including ischemia, stenosis, retraction, prolapse, parastomal hernia, peristomal skin erosion, and pouching difficulties.
Ischemia arises when the arterial blood supply is compromised by aggressive ligation of the adjacent mesentery, inappropriate division of vascular arcades, or by excessive tension on the mesentery. Superficial ischemia results in congestion and sloughing of the mucosa, but rarely requires surgical intervention. Full-thickness necrosis above the fascia may lead to stenosis and retraction of the stoma below the skin. Dilatation or local revisional surgery should be performed if evacuation difficulties arise. Recalcitrant stenosis or deep stomal retraction may necessitate a laparotomy to adequately mobilize the preceding bowel and revise the stoma. Urgent reoperation is warranted for necrosis that extends below the fascia, in order to stanch perforation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






