Chapter 40 Complications of First Entry into the Peritoneal Cavity
For laparoscopic procedures on the abdomen, initial entry usually entails gaining access to the peritoneal cavity so that carbon dioxide can be insufflated. This initial entry into the operative space is a necessary but potentially risky maneuver in minimally invasive surgery. In the normal state, the peritoneal cavity is a potential space, so successful insertion of an insufflation device necessitates insinuation of the device between contiguous organs and structures without causing injury. Depending on the type of procedure, first entry complications can account for up to 40% to 50% of all complications associated with minimally invasive surgery and up to half of the fatalities1,2 and can produce a serious medicolegal problem.1,3–7 A variety of techniques have been developed to obtain initial access into the peritoneal cavity, all of which have been associated with well-described complications. This chapter describes the basic devices and techniques available for peritoneal first entry, reviews the safety record of the common techniques, outlines the management of complications associated with these techniques, and describes the general operative steps in the performance of these techniques. Trocar-related issues that will not be covered in this chapter include port-site hernia, port-site metastasis, injuries related to insertion of secondary trocars, and complications of access for extraperitoneal or retroperitoneal procedures.
Level of evidence for recommendations
Unless otherwise stated, recommendations made in this chapter are based on data from uncontrolled clinical series and poorly designed trials. The strength of this type of recommendation, sometimes known as the recommendation’s grade or level of evidence, has been described by various authorities as “suggestive” (Agency for Healthcare Research and Quality [AHRQ]),8 “low” (AHRQ Evidence-Based Practice Center),9 “level C” (U.S. Preventive Services Task Force),10 or “grade C” (Oxford Centre for Evidence-Based Medicine).11
Current devices in use for first entry into the peritoneal cavity
Pneumoperitoneum Needle
The pneumoperitoneum needle in general use today originally was described in 1938 by János Veress in Kapuvár, Hungary, as a device to induce pneumothorax.12 The technique of peritoneoscopy (or celioscopy, or laparoscopy) already had been developed in the early 1900s. The method to gain initial access to the peritoneal cavity back then typically involved (1) direct insertion of a conventional needle to insufflate air into the peritoneal cavity or (2) a surgical cutdown to the peritoneum, with insertion of a needle under direct vision.13 Subsequent use of laparoscopy with the Veress needle slowly increased during the 20th century, and by the 1970s, the procedure was commonly used by gynecologists (and some gastroenterologists) for diagnosis, tubal ligations, and other minor procedures. At this point, the Veress needle was a common, if not the most common, technique of initial peritoneal access.
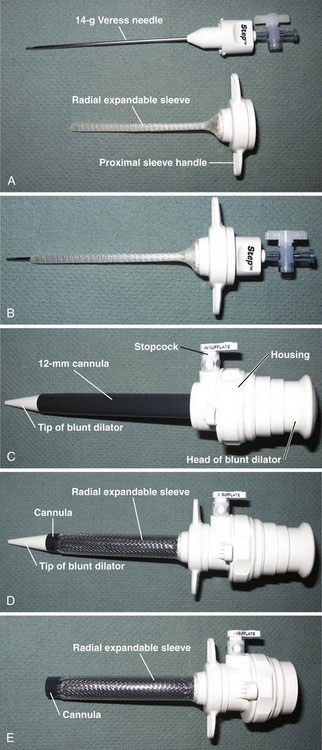
The design of the modern Veress needle (Figs. 40-1 and 40-2A) is similar to the original description,12 consisting of an approximately 2-mm hollow needle containing a spring-loaded obturator. The obturator retracts when the needle tip is applied to relatively immobile tissue, permitting the needle to cut a path. After the needle has passed through the immobile tissue and resistance decreases (e.g., the tip enters the peritoneal cavity), the obturator springs back into position, protecting the needle tip. After insertion into the abdomen, gas is insufflated through the needle-obturator assembly, typically through a Luer-Lok connection. In the mid-1990s, there was an effort to produce an “optical” Veress needle,14 which contained a small-diameter fiberoptic system that could visualize insertion, similar to how current optical trocars function (see later). The optical quality of the Veress “needlescope,” however, was limited compared with that of 5- or 10-mm endoscopes. There have only been a few publications with the optical Veress system since its introduction.
A related device that employs the Veress needle is the radially expanding trocar system (see Fig. 40-2). Developed in the mid-1990s,15,16 this multicomponent device consists of a needle-sheath assembly (see Fig. 40-2A and B) and a dilator-cannula assembly (see Fig. 40-2C). The needle-sheath assembly is inserted into the peritoneal cavity as a conventional pneumoperitoneum needle would be (see full description given later), and the abdomen is insufflated. The needle is withdrawn, leaving the sheath in place, and the dilator-cannula assembly then is inserted through the sheath. The sheath is an expandable polymer and accommodates the dilator-cannula as it is inserted (see Fig. 40-2D). After insertion, the dilator component is withdrawn (see Fig. 40-2E), leaving the 12-mm cannula (still within the sheath) for laparoscopic instrumentation. This placement method is somewhat reminiscent of the Seldinger technique used for central venous lines or enterostomy tubes. The purported advantages of a radially expandable system include less abdominal wall trauma (including a smaller fascial incision) compared with nondilating trocar systems, and the lack of need for suture of the fascial defect incurred by the device. Small randomized trials comparing the radially expanding trocar to nonexpanding trocars have shown advantages of the former, but these studies used the Veress needle for initial access in all patients.17,18
Open Laparoscopy
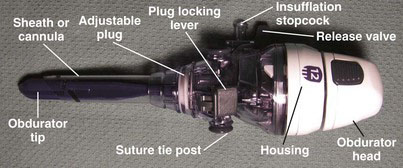
Associated with the widespread use of the Veress needle in laparoscopic surgery were rare but potentially devastating bowel and vascular injuries (see Safety Review of Techniques Used to Access the Peritoneal Cavity). These injuries occurred both with the insertion of the pneumoperitoneum needle and with subsequent insertion of the primary trocar—both of these maneuvers, of course, are performed blindly. In an attempt to circumvent this problem, Harrith Hasson, a gynecologist at the Grant Hospital in Chicago, introduced a blunt trocar device in 1971.19,20 Today, versions of this blunt trocar are generically known as the Hasson cannula (Fig. 40-3), which consists of a blunt obturator sheathed within a cannula. This device typically is inserted into the peritoneal cavity under direct vision with a cutdown at the umbilicus (i.e., a minilaparotomy). The cannula has a cone-shaped plug that helps form a seal against the fascial incision; the cannula is held against the abdominal wall with stay sutures that are wrapped around the suture tie posts. The technique of Hasson cannula insertion also is known as open laparoscopy (Veress needle access subsequently became known as closed laparoscopy). The purported benefits of open laparoscopy include the prevention of vascular injury, gas embolism, and extraperitoneal insufflation and the ability to enter an area with adhesions.
Optical Trocar
Background
In addition to the peritoneal access methods of Veress needle insertion and open laparoscopy, direct trocar insertion without prior pneumoperitoneum was described in 1978 by James Dingfelder, a gynecologist at the University of North Carolina21; further descriptions of direct insertion appeared in the gynecologic literature during the 1980s.22–24 Dingfelder’s rationale for direct trocar insertion (as opposed to Veress needle insertion) was to avoid potentially fatal complications of Veress needle misadventures.21 In addition, he noted that the historical assumption that pneumoperitoneum absolutely had to be established before trocar insertion did not have supporting evidence. As originally described, direct trocar insertion was a blind access procedure; the ports of the 1970s and 1980s did not have optical capabilities. Similar to Veress needle insertion, the success of direct trocar insertion was dependent on a sharp instrument and the skill of the surgeon. Limited controlled data did not find a difference in safety between the Veress needle and direct trocar insertion techniques.25,26 For all practical purposes, however, nonoptical direct trocar insertion was rendered obsolete by the advent of optical trocar technology in the 1990s.
In 1993, Steven Kaali, a gynecologist in Dobbs Ferry, New York, described an “Opti-Trocar,”27 which consisted of a conventional trocar with a transparent cutting tip and a pistol-grip handle. With an endoscope housed within the trocar as it was inserted, the surgeon could view the process of abdominal wall penetration in real time. Kaali did not perform direct insertion with this device, but rather established pneumoperitoneum first with a Veress needle. Also in 1993, Andreas Melzer’s group in the Department of Surgery at the University of Tübingen, together with the Olympus company, described an “optical scalpel,” a trocar with an externally controlled cutting tip that accommodated an endoscope.28 This device also permitted visual monitoring of transabdominal penetration. Around the same time, a port with a clear conical tip was developed by Riek and colleagues, also at the University of Tübingen29; this was the forerunner of Ethicon’s Xcel trocar. In addition, U.S. Surgical developed the Visiport device during the early 1990s.30 Since the late 1990s, a number of series have been published supporting the safety and efficacy of the optical trocar.31–36
Endopath Xcel
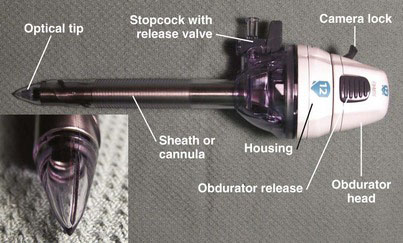
The Endopath Xcel Bladeless Trocar (Ethicon Endo-Surgery, Cincinnati, Ohio) (Fig. 40-4) has an obturator with a transparent conical plastic tip, which has an edge that is sharp enough to induce tissue separation during insertion. An endoscope is inserted through the obturator head, allowing the surgeon to monitor insertion progress in real time. This trocar comes in a range of diameters between 5 and 15 mm (and bariatric lengths) and may be used for direct insertion with a 5- or 10-mm laparoscope. The precise definitions of bladed and bladeless with this trocar are somewhat overlapping because, if enough pressure is used, the plastic conical tip of the Xcel can cut through tissue.
Visiport Plus
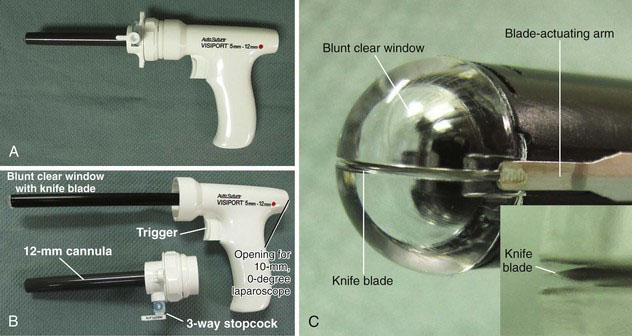
The Visiport Plus Optical Trocar (Fig. 40-5) (Covidien, Norwalk, Conn.) consists of a conventional 10-mm cannula through which a pistol-shaped obturator (see Fig. 40-5B) is inserted. The obturator has a clear blunt tip that houses an actuated knife blade (see Fig. 40-5C). With each depression of the device trigger, the knife blade is fired out 1 mm and quickly retracts; the actual motion of the blade cannot be detected with the unaided eye. With the laparoscope inserted through the obturator and the obturator within the tissue of the abdominal wall, the surgeon fires the blade and watches the progress of the tip penetration on the video screen. Of note, the Visiport’s product insert strongly recommends that pneumoperitoneum be established before entering the peritoneal cavity with this device; that is, the manufacturer does not recommend that the Visiport be used as a direct insertion device. Of the four optical ports described in this section, the Visiport is the only one that is “bladed.”
Kii Fios First Entry
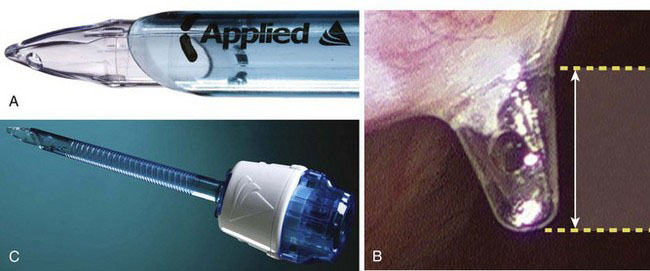
Applied Medical (Rancho Santa Margarita, Calif.) released the Kii Fios First Entry System (Fig. 40-6) in 2008. This is an optical trocar intended for direct insertion without prior pneumoperitoneum. The concept is similar to the Ethicon Xcel trocar, except the Applied Medical device has a port in the distal tip (see Fig. 40-6B and C), which allows relatively rapid insufflation of the peritoneal cavity with just minimal tip penetration (about 3 mm) into the peritoneal cavity. Per the company’s website (www.appliedmedical.com), the Kii Fios First Entry device is the only device with U.S. Food and Drug Administration 510(k) documentation for first entry use. This device comes in 5- to 12-mm diameters, with bariatric lengths.
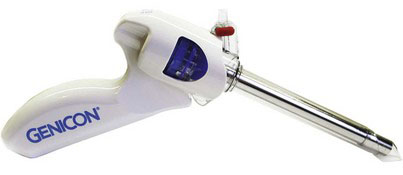
Genicon Bladeless Tip Trocar
The Genicon (Winter Park, Fla.) Bladeless Visual Tip Trocar and Cannula System (Fig. 40-7) was released in 2005. This system consists of an obturator with a transparent conical tip mounted to a pistol-grip; the obturator assembly is inserted through a cannula (available in 5- to 15-mm diameters, with bariatric lengths). The plastic optical tip permits dissection through tissue under direct endoscope visualization, similar to the Ethicon Xcel and the Applied Medical Fios devices mentioned earlier.
Karl Storz EndoTIP
The Karl Storz (Tuttlingen, Germany) Ternamian EndoTIP trocar (Endoscopic Threaded Imaging Port; Fig. 40-8) is an optical device that is designed to be screwed into the abdomen.37 The purported advantage to this device is that less axial force is needed during insertion compared with nonthreaded ports. This device is not intended for primary insertion before pneumoperitoneum, but rather for insertion after pneumoperitoneum has been established (or for secondary insertion after a laparoscope has been positioned).38 The description of the EndoTIP is included here for completion.
Safety review of techniques used to access the peritoneal cavity
Search Strategy
The following terms were searched using Google Scholar (http://scholar.google.com): pneumoperitoneum complications, laparoscopy complications, trocar injury, trocar insertion, closed laparoscopy, open laparoscopy, laparoscopic access, and laparoscopic entry. In addition, specific device names (e.g., Xcel, Visiport, optical trocar, VersaStep, Veress, Hasson, radially expanding trocar) were searched. The first 300 results (hits) of each term were reviewed, and the full-text version of each relevant article was retrieved. In addition, bibliographies of major articles were reviewed for further relevant material. Many more articles were identified with the above search strategy than were actually referred to in this chapter. Most of the nonreferenced articles were comparatively small (<500 cases) studies on peritoneal access, either as the primary issue or as component of a general series. For the most part, the data on these smaller studies already have been cataloged and analyzed in one or more systematic reviews; such an exercise was not repeated for this chapter. Instead, the data as collected by these reviews have been summarized here.
Major (Retroperitoneal) Vascular Injury
Vascular injury during insertion of the Veress needle, particularly in the infraumbilical position as is commonly done for gynecologic procedures, has long been known to have a small risk for injury to major vascular structures. Three fourths of these injuries are arterial, and of these, the aorta is the most common site.39 In fact, this risk for vascular injury was one of the driving factors for development of the blunt (Hasson) cannula for open laparoscopy.40 The rates of major vascular injury for various insertion methods, as described in large systematic reviews, are shown in Table 40-1. Essentially, use of the Veress needle can be expected to result in a major vascular injury in one of every 2000 to 10,000 patients. Not surprisingly, the rate of vascular injury secondary to the pneumoperitoneum needle has been higher in controlled studies compared with retrospective studies (1.8 vs. 0.3 per 1000), suggesting that there may be some underreporting of these injuries in retrospective studies.41
Table 40-1 Incidence of Major Vascular Injury Associated with First Entry in Laparoscopic Surgery, as Obtained from Systematic Reviews, Large Series, or Surveys
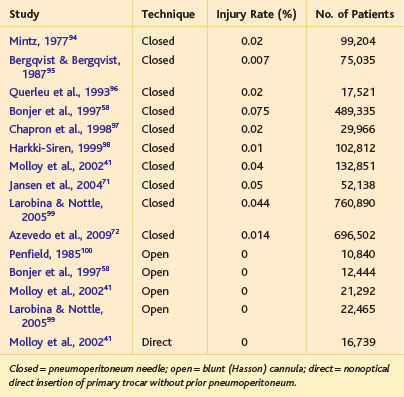
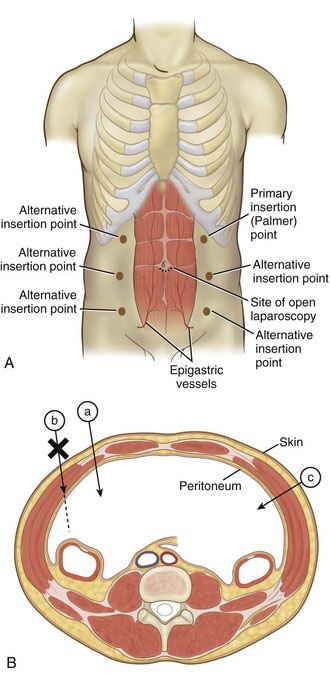
The Table 40-1 data, as mentioned earlier, largely are derived from the infraumbilical insertion technique. It is suspected, but not yet know with surety, that Veress needle insertion in the left upper abdomen (e.g., at the Palmer point42; Fig. 40-9) will reduce the incidence of vascular injury secondary to this instrument.43,44 In contrast, use of open laparoscopy with the infraumbilical approach all but eliminates the risk for vascular injury. A 2008 Cochrane review calculated a statistically higher incidence of vascular injuries associated with closed laparoscopy compared with open laparoscopy.41 There have been some case reports of vascular injury with open laparoscopy,45–47 but these have been secondary to gross technical error (e.g., nicking the aorta with the scalpel during the skin incision) or defective equipment.
In a review of 502 vascular injuries associated with the closed laparoscopy,2 46% of the injuries were secondary to the Veress needle itself, and 54% were secondary to the subsequent insertion of the primary trocar (also a blind maneuver). Of course, being confident of which instrument caused an injury when both are inserted blindly may not seem reasonable, but it probably is safe to say that Veress needle injuries actually represent a mix of both needle and primary trocar injuries. With respect to optical trocar insertion, the true incidence of vascular injury48 is difficult to know because the recorded patient numbers are not as high as with closed and open laparoscopy. In addition, the optical trocar safety data are somewhat diluted because systematic reviews may include data from studies that used the older technique of direct (nonoptical) insertion.41,49
The mortality related to a major vascular injury incurred during first entry into the peritoneal cavity has not been high; in the 2008 Cochrane review on peritoneal access, five deaths were noted, all associated with use of the Veress needle, but none of these was secondary to vascular injury. Major cardiovascular collapse during Veress needle insertion may be secondary to gas embolism and should not be attributed immediately to hemorrhage. In addition, the pattern of delayed mortality from an unrecognized bowel injury does not appear to have an analog in major vascular injury; for instance, delayed rupture of a traumatic pseudoaneurysm is exceedingly rare.50,51 Most (90%) major vascular injuries incurred during initial access are recognized at the index operation.41,52,53 The patient who does develop a pseudoaneurysm after laparoscopy usually has a history of Veress needle access; presentation can occur weeks to months later with pain and gastrointestinal bleeding.54
Intestinal Injury
Historically, nearly half of bowel injuries associated with laparoscopic procedures were incurred during peritoneal access.55 With an increasing number of complex and reoperative abdominal procedures being performed in minimally invasive surgery, the proportion of bowel injuries secondary to access likely will decline. The absolute rates of bowel injury associated with various entry techniques is shown in Table 40-2. Unlike the situation with vascular injury, both closed and open laparoscopy have a defined incidence of bowel injury; some believe that the open (Hasson) technique may have a higher risk for this injury.41,56 The incidence of bowel injury associated with optical trocar insertion is in the range of 0.05%, although there are fewer data. Essentially, use of any of these techniques can be expected to result in an intestinal injury in 1 of every 1000 to 2000 patients. Importantly, however, intestinal injury associated with Hasson insertion is recognized more frequently than with Veress needle insertion, which can reduce morbidity and mortality secondary to injury from the former device.57
Table 40-2 Incidence of Intestinal Injury Associated with First Entry in Laparoscopic Surgery, as Obtained from Systematic Reviews, Large Series, or Surveys
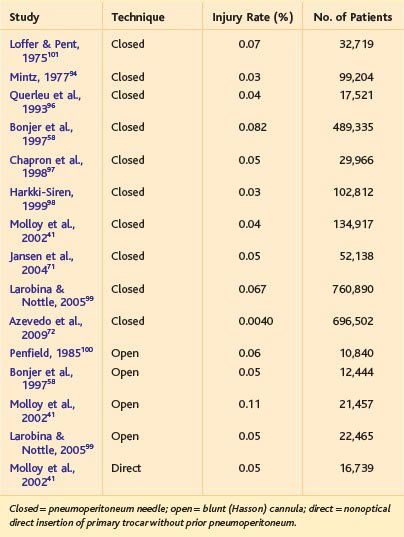
Intestinal injury incurred during peritoneal access is more common in general surgical than in gynecologic patients (1.5 vs. 0.4 per 1000)41; the reason is not immediately obvious but may be secondary to an increased prevalence of prior abdominal procedures in general surgical patients. In a series of 430 previously published bowel perforations (not all associated with the initial entry),55 the small intestine was the most common site of injury (56%), followed by the colon (39%). With respect to intestinal injury associated with the pneumoperitoneum needle, most (about 90%) perforations have been presumed to be caused by the blind insertion of the primary trocar, and not the needle per se.41 Comparing these data to vascular injury, a greater percentage of intestinal injuries has been attributed to insertion of the primary trocar, rather than the Veress needle itself.
The mortality rate of entry-related intestinal injury can range up to 3% to 5%,55,57 or about 1 mortality for every 20 bowel perforations. Most of these deaths are the result of a missed (i.e., undiagnosed at the index procedure) injury that produces delayed intra-abdominal sepsis.4 A review by one group found that only one third of intestinal injuries associated with minimally invasive procedures, whether incurred during the access phase or operative phase, were diagnosed during the operation57; a review by another group found that bowel injury was diagnosed at operation or within 24 hours in two thirds of the cases.55 In the 2008 Cochrane review of peritoneal access,49 two of the five deaths reported were secondary to unrecognized intestinal injury, both from the Veress needle technique.
Gas Embolism
Gas (CO2) embolism during first entry into the peritoneal cavity has been a complication primarily of the Veress needle, presumably from insertion and insufflation into a major venous structure. There have been no reported cases of gas embolism incurred during access with open laparoscopy.39 Of course, gas embolism may occur (rarely) during the course of most laparoscopic procedures, possibly through the lumen of a vein that was opened during the course of the dissection. Intravascular insufflation associated with insertion of a Hasson cannula, however, does not appear to be possible. The incidence of gas embolism (also known as intravascular insufflation) associated with insertion of the pneumoperitoneum needle in a systemic review of nearly 500,000 closed laparoscopies58 was 0.0014% (7 cases), or about 1 in every 70,000 cases. The mortality rate in these 7 cases was 100%. In contrast, the mortality rate from gas embolism in a separate case series was only about one third (2 of 7 cases).59
Other Injuries
Although vascular and visceral injury remain the most studied and discussed complications associated with first entry into the peritoneal cavity, other less severe injuries can occur, such as abdominal wall hematoma (trocar site bleeding) and extraperitoneal insufflation (not intravascular). The precise incidence of trocar site bleeding is difficult to specify (which may be due in part to underreporting of minor complications) but has been estimated to be 0.2% to 2% of laparoscopic procedures.60
Abdominal wall hematoma primarily is incurred at lateral insertion sites (see Fig. 40-9); laceration of the epigastric vessels is a common cause. Computed tomography mapping of the epigastrics demonstrated that they run in a region 4 to 8 cm lateral to the midline.61 A hematoma generally should not occur with device insertion at the umbilicus, unless the operator veers off the midline. With respect to prevention of abdominal wall hematoma, there may be an advantage with the use of radially expanding access devices (see Fig. 40-2), which were associated with fewer episodes of trocar bleeding in a Cochrane review compared with nondilating trocars.49
Extraperitoneal insufflation can occur during the course of most laparoscopic procedures, but that associated with first entry devices appears to be more common with the use of the Veress needle.49 The incidence of extraperitoneal insufflation, similar to abdominal wall hematoma, has been difficult to know, presumably because of underreporting.
Failed Entry
Failure to gain access to the peritoneal cavity is another complication of initial entry, but one that usually is not associated with a clinically important injury. The incidence of failed entry also has been difficult to establish. Part of this problem may be secondary to the definition of failure, which in any given patient might be defined as failure at the first attempt to gain access with success on a subsequent attempt, to complete inability to gain access after multiple attempts, with abandonment of the technique and migration to an alternative operative plan. Underreporting also is likely to be an issue in determining the incidence of access failure because this complication in isolation can be perceived as a purely technical failure with minor impact on patient outcome; accordingly, some surgeons may not see any relevance in reporting failed entry. Nevertheless, the 2008 Cochrane review of peritoneal access methods concluded that direct trocar insertion was associated with a decreased rate of access failure compared with the Veress needle insertion.49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree