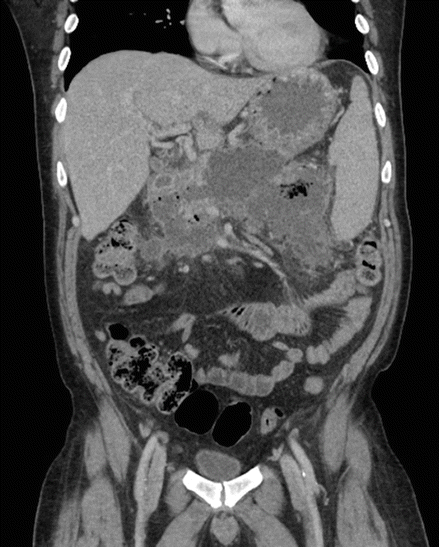
Figure 7.1
CT scan of the abdomen with extensive pancreatic necrosis, with a large amount of peripancreatic edema and several poorly organized fluid collections, the largest measuring 5.7 cm × 2 cm × 2.7 cm

Figure 7.2
MRCP showing a disrupted pancreatic duct at the level of the pancreatic mid neck
My Management
- A.
Correctly identify the type of fluid collection.
- B.
Initiate a multidisciplinary evaluation that includes gastroenterology, interventional radiology, and surgery.
- C.
Anticipate medical management followed by reevaluation for interval endoscopic transmural drainage/debridement.
Diagnosis and Assessment
Acute pancreatitis (AP) begins with an intense inflammatory process within the pancreas that may extend into surrounding tissues. This inflammatory process may also lead to a number of local complications within or around the pancreatic tissue, including the formation of fluid collections, major vascular or bleeding complications, and pancreatic duct disruption.
As per the Atlanta Classification System revised in 2013, fluid collections have been organized into four general categories: (1) acute peripancreatic fluid collections (APFC), (2) pancreatic pseudocysts (PP), (3) acute necrotic collections (ANC), and (4) walled-off necrosis (WON) [1]. These complications represent a progression of local injury that begins early during a patient’s episode of AP. There may be early indicators on imaging that local complications will develop; however, these findings and the subsequent development of local complications alone do not imply the patient will experience a severe clinical course [2]. Acute peripancreatic fluid collections, when present, appear early during acute pancreatitis (i.e., within 4 weeks by definition) and generally resolve without major sequelae [3]. These are non-necrotic collections without a discrete wall, are generally confined by fascial planes, and usually remain sterile. Acute necrotic collections may also develop within the first 4 weeks and are due to necrosis of the pancreas and/or peripancreatic tissues. In very early phases, it may be difficult to differentiate ANC from APFC; however, ANC are usually fairly distinct outward of 1 week from acute pancreatitis and appear as heterogeneous collections with various amounts of semisolid and necrotic debris. Walled-off pancreatic fluid collections are delayed sequelae that usually take over a month to develop and as a group include pancreatic pseudocysts (PP) and walled-off necrosis (WON). Pancreatic pseudocysts represent collections of simple fluid and in general are thought to form in communication with the pancreatic duct system. On the other hand, WON represents an encapsulation of necrotic material within an inflammatory wall that can take weeks to fully mature. On contrast enhanced CT, the wall is often enhancing, but the contents may not always be easily characterized as solid or simple fluid, which may explain why some of these are mislabeled as pseudocysts.
Both ANC and WON may be sterile or become infected, and this may become apparent clinically or on imaging showing gas formation within the collection. In general the diagnosis of infected necrosis can usually be made clinically, although sometimes it can be challenging to differentiate infection from the systemic inflammatory response of necrotizing pancreatitis alone. Sampling the fluid either percutaneously or endoscopically is one way to help establish the diagnosis of infection when in question, but is typically not necessary or desired, having both a low negative predictive value and associated risk of contamination or inoculation [4].
Major vascular complications and hemorrhage develop in a minority of patients with acute pancreatitis. The inflammatory process and proteolytic activity of pancreatic enzymes can injure both local arterial and venous vasculature [5]. Early in a course of acute pancreatitis, these effects have the potential to disrupt the integrity of vessels and cause diffuse pancreatic hemorrhage or bleeding from a major artery in proximity to the pancreas. These events are rare, occurring in around 0.5% of acute pancreatitis cases [6]. Delayed bleeding, occurring 2 months beyond one or more episodes of acute pancreatitis, is also uncommon and may occur in 1% of cases [7]. The more common sources of these delayed bleeding events are bleeding pseudoaneurysms, diffuse bleeding from pancreatic necrosis, and hemorrhagic pseudocysts. Many of these events can be managed through vascular embolization, but others may require operative care. Peripancreatic venous thromboses also develop in some patients with acute pancreatitis and may result from venous stasis related to mass effect from edema or direct damage to the vessels from the inflammatory milieu [8]. It is not clear from available evidence whether patients should be routinely anticoagulated for this condition.
Acute pancreatitis may also result in pancreatic duct disruption (PDD) and leakage of pancreatic secretions. Some of these leaks resolve spontaneously or with conservative medical management, but they may also persist and lead to pancreatic pseudocysts, internal pancreatic fistulae (IPF) with pancreatic ascites or effusions, or external pancreatico-cutaneous fistulae (EPF). In patients with acute necrotizing pancreatitis, the disruption is often complete, meaning that the pancreatic duct proximal to the leak does not opacify on endoscopic retrograde pancreatography (ERP), and is referred to as a disconnected pancreatic duct syndrome (DPDS). The disruption may also only be partial, and evaluation should include assessment for pancreatic duct stricture and calculi, which may impact the approach to therapy. Most of this can now be accomplished noninvasively using contrast enhanced CT or MRCP with or without secretin stimulation, and unlike ERP, these imaging techniques can characterize the anatomy proximal to a complete disruption.
The management and outcome of PDD depends largely on the pancreatic duct anatomy and type of complication that develops as a consequence of duct leakage [9]. Conservative medical management alone is an option in many cases [10], and small asymptomatic pseudocysts often do not require intervention [11]. When intervention is required, the approach may involve a combination of endoscopic, radiologic, or surgical procedures.
There are few comparative effectiveness trials that have established the preferred management strategy for pancreatic pseudocysts, and selection of technique is usually handled on a case by case basis. However, endoscopic treatment appears to be more effective than percutaneous drainage and is at least as effective and associated with less morbidity and lower costs than surgery [12, 13]. Endoscopic therapy often involves transmural drainage of the fluid collection with stenting across an endoscopic cystogastrostomy or cystenterostomy. While not always practiced, use of endoscopic ultrasonography (EUS) for these procedures has certain advantages and can be generally suggested. Importantly, transmural drainage does not necessarily imply that the disruption will heal, and there is still a risk that the fluid collection will recur once the transmural stents are removed [14]. This is a particular problem in patients with DPDS who may require long-term or permanent transmural stents.
Endoscopic treatment may also involve transpapillary access of the pancreatic ducts and fluid collections. This technique can allow for direct drainage of the collection or be used to place a pancreatic duct stent to redirect the flow of secretions. There is some uncertainty about the efficacy of this approach and concerns about poorer outcomes when used in combination with transmural drainage [15, 16], but it remains an option when a transmural approach is not technically feasible. The benefit may be limited to some cases of partial disruption, and in general the aim should be to bridge the defect with the stent [17, 18]. It is also not clear how long the stent should be left in place, and there is potential for these stents to cause duct injury [19].
In a minority of cases, pancreatic duct disruption can lead to internal fistula and the development of pancreatic ascites and pleural effusions. Medical management in these cases may not be adequate and surgery is associated with significant morbidity and mortality [20]. Alternatively, endoscopic therapy with pancreatic sphincterotomy and stenting may be an effective option that can provide durable results [21]. External pancreatico-cutaneous fistulae are also encountered infrequently and have traditionally been managed medically, with surgery reserved for refractory cases or those unlikely to resolve spontaneously. Similar to IPF, endoscopic therapy with transpapillary stenting has been used for some of these cases and may also be successful [22].
Management
The patient presented above returned several weeks after an episode of acute necrotizing pancreatitis with severe pain and organizing fluid collections. His symptoms were controlled medically, and follow-up imaging in 1 month showed stability in the size and extent of the collections. He underwent an elective ERP demonstrating complete pancreatic ductal disruption at the mid neck without identification of a proximal remnant, and a pancreatic duct stent was placed into the duct to the site of disruption. He returned a couple of weeks later with fever and chills, and a CT showed enlarging walled-off pancreatic fluid collections with air-fluid levels (Fig. 7.3). Endoscopic cystogastrostomy and placement of a 15 mm lumen apposing covered self-expanding metal stent (LACSEM) was then performed, and copious pus and debris was seen draining across the stent into the stomach.