Intraepithelial neoplasia
Intervals for follow-up endoscopy
None
2–3 years
Low grade
6 months, then every 12 months as long as low-grade dysplasia persists
High grade
Mucosal irregularity
Endoscopic resection
Inconspicuous mucosa
3 months surveillance
Pathology confirmation, and repeat biopsies
If HGIN confirmed
Endoscopic resection
7.2.1 Endoscopic Standards for Barrett’s esophagus
The endoscopic diagnosis of Barrett’s esophagus and associated neoplasia requires a standardized endoscopic approach [7, 9]:
Barrett’s esophagus:
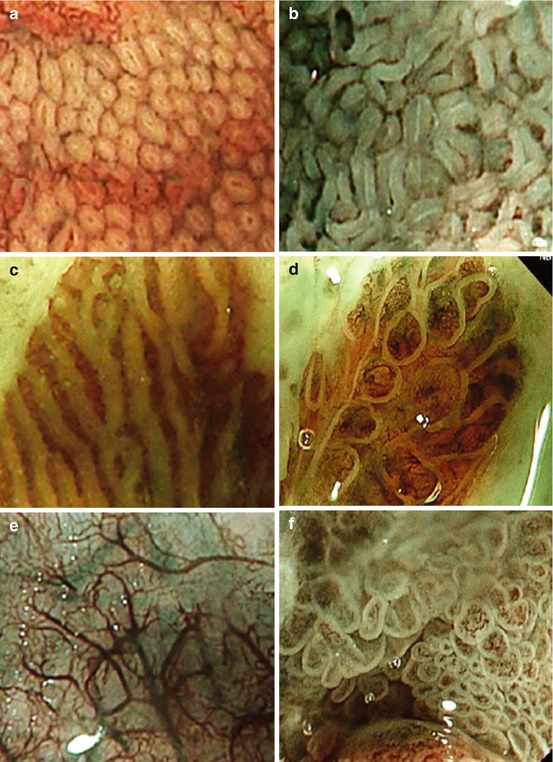
The Prague or CE classification describes the amount of metaplastic epithelium within the lower esophagus (Fig. 7.1).


Fig. 7.1
(a, b) Barrett esophagus C2 M3, according to the Prague classification (circular extent 2 cm, maximum extension 3 cm). Do not mistake the diaphragmatic hiatal impression (circle) for the tops of the gastric folds (gastroesophageal junction at end of white marking lines) [Pentax, WLI]. (c) i-Scan imaging of the two presentations of SIM in BE that are (d) villous/ridge pits with regular microvasculature and (e) absent pits with regular microvasculature [Pentax, i-scan, ~50-fold]
The key steps are:
Identify the gastroesophageal junction as at the tops of the gastric mucosal folds (Western definition) or distal end of esophageal palisade vessels (Asian definition) [2].
If hiatus hernia is present, do not mistake the diaphragmatic hiatal impression for the gastroesophageal junction (Fig. 7.1).
For circumferential columnar-appearing mucosa above the gastroesophageal junction, define this extent in centimeters above the gastroesophageal junction: report as the C value.
For any tongue-like areas of columnar-appearing mucosa, measure the maximum extent in centimeters above the gastroesophageal junction: report as the M value.
Barrett’s associated neoplasia:
Surveillance endoscopy for BE should be performed after a 4-week course of proton pump inhibitor therapy for better detection of Barrett’s extent and neoplastic lesions, in particular when using chromoendoscopy for detection of SIM (sensitivity 98 %, specificity only 61 % without PPI pretreatment) [14].
7.2.2 Detection of Specialized Intestinal Metaplasia (SIM) vs. Gastric-Type Epithelium
Several enhanced imaging techniques can be used to differentiate between SIM and gastric-type epithelium within columnar-lined lower esophagus (Table 7.2; Fig. 7.2a–d). Chromoendoscopy with acetic acid, indigo carmine (or methylene blue), can be used to unmask villous- or gyrous-type mucosal pattern.
Typea | Surface pattern | Histopathology | Fig. no. |
|---|---|---|---|
RMSP | Uniform pits | Columnar-lined mucosa: | |
Small round | “Fundus” type | ||
Slit-like oval | “Corpus” type | ||
RMSP | Uniform | Normal columnar epithelium, LBC = intestinalized (SIM)b | |
Tubular | |||
RMSP | Uniform | Normal columnar epithelium | |
Linear/ridges (top) | “Cardia ~” | ||
Villous (bottom) | “Antrum ~” | ||
−/+light blue crests LBC (on NBI) | LBC = apical brush border (intestinalized cells) (SIM)b | ||
AMSP | Absent MSP (atrophic mucosa) with arborized SMVsc | “Flat intestinal metaplasia” (SIM)c | |
IMSP (and IMVP) | Irregular MSP micrified villous/gyrous, irregular WOS, no demarcation; irregular MVP | HGIN/carcinoma M1 | |
IMSP and IMVP | Severely irregular MSP with destroyed pits/fused villi & demarcation; severe IMVP with sparse/thick microvessels | Carcinoma, likely sm invasive |


Fig. 7.2
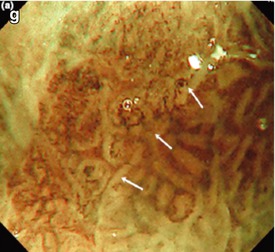
Surface patterns of esophageal columnar epithelium (NBI). Permission by Blackwell Publ. Ltd. [12]. (a) Normal fundus-type columnar-lined mucosa (uniform round pits). (b) Uniform tubular-type Barrett epithelium with LBC (indicating SIM). (c) Uniform cardia-type columnar epithelium with LBC (Barrett’s mucosa). (d) Uniform villous-type columnar epithelium with LBC (Barrett’s mucosa). (e) Columnar epithelium with absent MSP (AMSP), arborized submucosal veins (SMV). (f) Irregular micrified villous MSP with irregular WOS, typical of HGIN/M2 carcinoma (g) Severely irregular IMSP and IMVP, fused villi, indicative of sm-invasive adenocarcinoma. OLYMPUS Lucera, M-NBI (100-fold)
Acetic acid shows accuracy between 52 and 92 %. Methylene blue seems not to be efficient identifying SIM based on a meta-analysis from 2009 [15]. Few studies indicate usefulness of indigo carmine for the detection of SIM (accuracy 71–97 %) [9, 16].
7.2.3 Detection of Neoplasias in Barrett’s Esophagus
The majority (85 %) of early malignant Barrett’s neoplasias are diminutive and flat lesions (0-IIa,b,c) and are difficult to detect [19, 20].
Methylene blue staining seems not to be effective diagnosing Barrett’s associated neoplasia [13, 15]. However, acetic acid-enhanced magnification endoscopy can be used to unmask irregular microsurface pattern (MSP) of subtle neoplastic areas (Table 7.2; Fig. 7.2f, g). The surface pattern is best visualized after spraying of 1.5 % acetic acid. The MSP of neoplasias is irregular villous/gyrous, often micrified (Fig. 4.12b, d) or nonstructured with irregular MVP, often mixed with remnants of type I pattern or squamous epithelium [21]. NBI in combination with magnification also visualizes the microvascular pattern (MVP) of the mucosa [16]. Characteristic NBI features of Barrett’s associated neoplasias are distorted microsurface with irregular microvasculature [12, 17] (Table 7.2; Fig. 7.2f, g and 7.3).
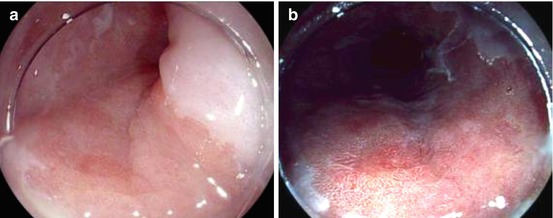

Fig. 7.3
Barrett esophagus, depressed lesion type 0-IIc with microinvasion of the submucosa, irregular pit pattern [Pentax WLI (left) and i-scan (right), ~20-fold]
Overall, enhanced magnification endoscopy including inspection with NBI achieves about 80 % sensitivity for detection of HGIN and early adenocarcinoma in BE in experienced hands [22].
7.2.4 Endoscopic Diagnosis of HGIN/sm-Microinvasive Cancer vs. Deeply sm-Invasive Cancer in Barrett’s Esophagus
Nonstructured, irregular MSP combined with irregular MVP probably predicts deeply invasive cancer in BE [21], but the diagnostic accuracy has not yet been proven for deep submucosal invasion in early Barrett’s cancer (Table 7.2).
Regular MSP of Barrett epithelium has well been characterized by the group in Nottingham using the Japanese RBG-rotating wheel imaging system (comp. Chap. 4) with superior image resolution as compared to standard Western imaging systems (Fig. 7.2, RMSP and AMSP images reduced in size, from Nottingham classification [12], permission granted by Blackwell Publishing Ltd.). So far, the diagnostic accuracy of the classifications for Barrett’s mucosa and neoplasias has not been prospectively evaluated.
However, the association has been convincing between deep submucosal invasion and macroscopic types of lesions [19] (Table 7.3). In a large prospective series of 380 Barrett’s neoplastic lesions, most of the HGIN lesions were type 0-IIb (70 %) and 0-IIa (17 %). The likelihood for submucosal invasive early cancer was lowest for lesions type 0-IIb (3 %) and progressively increased for lesions type 0-I (10 %), 0-IIa (14 %), IIa + c (18 %), and 0-IIc (24 %) [19]. Due to difficult recording at this location, high-frequency EUS (20–30 MHz) was not reliable enough to detect submucosal invasion in neoplastic Barrett’s lesions (sensitivity only 27 %) [23].
Table 7.3




Distribution of macroscopic types in early Barrett neoplasias and submucosal invasiveness and undifferentiated grading per typea
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








