Superficial neoplastic lesion
Prevalence (%)
Cancer risk (%)
Recommended resection
Polypoid 0-Ip/Isp/Is
~15–20
1–15
Snaring
Elevated/flat 0-IIa/b
~5
4–6
EMR
Depressed 0-IIc

~0.5
30–75
→ En bloc
Superficial neoplastic lesion | Prevalence | Cancer risk | Recommended resection | |
|---|---|---|---|---|
LST-G(H; homogenous) |  | ~5 | 0–1.5 | EMR |
LST-G(M; mixed nodular) |  | ~5 | 13(−30 a ) | → En bloc |
LST-NG(F, flat) |  | ~3 | >10(−29 a ) | → En bloc |
LST-NG(PD, pseudodepressed) |  | ~1.5 | 28– ~70 | → En bloc |
10.3 Basic Structure of Colonic Mucosa and Colorectal Neoplasias
Colorectal mucosa is covered with cylindrical cell epithelium containing absorptive colonocytes and mucin-producing goblet cells. On standard WLI, normal colorectal mucosa shows smooth surface reflex (of mucin layer) and mildly reddish color with branching (dendritic) submucosal vascular pattern of collecting venules (Fig. 10.1a, b). Colonic mucosal glands are tubular structures and the pitlike gland openings form a regular carpet of small round pits – named normal PP type I [5] (Fig. 10.2a). Inflammation causes mucosal edema and vascular erythema of mucosal and sm layer, diminished surface reflex (by inhomogenous mucin layer), and epithelial erosions with whitish fibrin exudates or even mucosal ulcers (Fig. 10.5, see below). Permeation of dendritic sm vascular pattern is diminished or absent, but surface shows normal round pits type I or, when chronic, regenerative hyperplasia with stellar pit pattern type II (Fig. 10.2b).
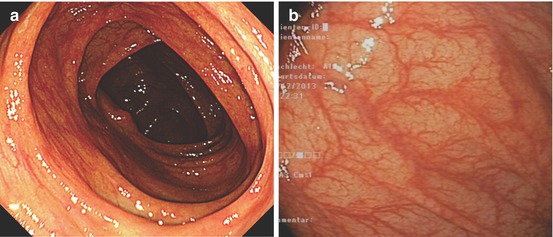
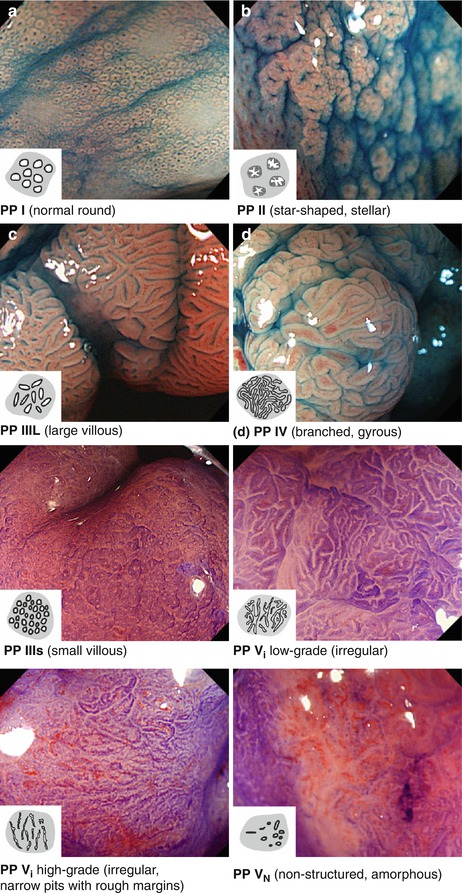

Fig. 10.1
(a) Normal ascending colon, WLI, and (b) normal ascending colonic mucosa, WLI

Fig. 10.2
Colonic pit pattern types I–Vn (According to Kudo [5, 16]). Magnified (~40–80-fold) chromoendoscopy (a–e, indigo carmine; f–g, crystal violet). Compare Table 10.2b for explanation. (a) PP I (normal round). (b) PP II (star-shaped, stellar). (c) PP IIIl (large villous). (d) PP IV (branched, gyrous). (e) PP IIIs (small villous). (f) PP VI low grade (irregular). (g) PP VI high grade (irregular, narrow pits with rough margins). (h) PP VN (nonstructured, amorphous)
Image-enhanced magnification endoscopy (IEE) with magnifying NBI and/or chromoendoscopy is used for analysis of early mucosal neoplasias. S. Kudo has first characterized magnified chromoendoscopic surface structure of glands (pit pattern) in normal mucosa and hyperplastic and neoplastic mucosal lesion in the colon (Table 10.2b), and Y. Sano using magnifying NBI endoscopy investigated alterations in capillary pattern of such lesions as compared to normfal mucosa (Table 10.2a). The Japan Gastroenterological Endoscopy Society has achieved consensus on classifications of capillary pattern (CP) and pit pattern (PP) [5, 11]. A simplified version of both is the Narrow-Band Imaging International Colorectal Endoscopic Classification (NICE) for standard endoscopy (indigo carmine and NBI) (Table 4.2) [11, 12]. But original CP and PP classification more accurately diagnose sm-invasive cancer.
Table 10.2a
*Colonic microvascular pattern according to Sano [11] (permission granted by John Wiley & Sons Inc.)
Type I | Type II | Type IIIA | Type IIIB |
|---|---|---|---|
 |  |  |  |
Meshed capillary vessels (−) (faint pattern) | Meshed capillary vessels (+) | Meshed capillary vessels characterized by blind ending, branching, curtailed irregularity | |
Vessel surrounds mucosal glands | *Lack of uniformity *High density of capillary vessels | *Nearly avascular or loose capillary vessels | |
Typea | Description of pits | Histopathological correlates | |
|---|---|---|---|
 | I | Round (uniform pits) | Normal or inflammatory mucosa |
 | II | Stellar or papillary | Hyperplastic mucosa (hyperplastic polyp or serrated adenoma) |
 | IIIsb | Small tubular, round | Adenoma or carcinoma (often depressed type) |
 | III l | Large tubular or round | Adenoma (often classical polypoid adenoma) |
 | IVa | Branching or gyrus-like | Adenoma (often villous) |
 | V I low-grade | Irregular pits with smooth margins | Adenoma (LGIN), early cancer (HGIN, T1m, or T1sml) |
 | V I high-grade | Irregular, narrow pits With rough margins | sm-invasive cancer (80 % ≥ sm2) |
 | V N | Nonstructured | sm-invasive cancer (≥sm2) |
Colonic Capillary Pattern (CP) (Table 10.2a). CP type I appears as scanty, regular reticular network in normal mucosa on M-NBI – reflecting the capillary meshwork around regular gland grooves. In hyperplastic lesions it is obscured by the hyperplastic marginal crypt epithelium and barely visible. Regularly meshed CP type II indicates adenoma, and irregular CP type IIIA pattern is seen in intramucosal or superficial submucosal extension of cancer and in rare lesions 0–IIc with undifferentiated adenocarcinoma (<2 % of colorectal carcinoma). CP type IIIB is sparse, is very irregular with few thick vessels, and suggests deeply sm2-3 invasive cancer (specificity 85 %) [11, 13, 14].
10.4 Differential Diagnosis of Macroscopic Type of Colorectal Lesions
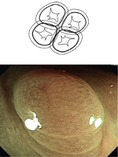
Mucosal neoplasias (adenoma, HGIN, adenocarcinoma) are lesions with clear margins and disappearance of dendritic submucosal vessel pattern and exhibit neoplastic pit patterns (III–V) with indigo carmine CE and/or variants of hyperplastic pit pattern (type II; below Fig. 10.15) in case of serrated adenomas (algorithm in Fig. 10.3). Detection of lateral margins of protruding or flat neoplasia is easy in normal colonic mucosa. Lack of clear margin in the presence of hyperplastic pit pattern favors hyperplastic (nonneoplastic) polyps (HP), most of them in rectosigmoid colon as lesions 0-Is/Isp or 0-IIa (Fig. 10.4a, b). They must not be confused with serrated adenomas that also exhibit hyperplastic pit pattern, often in right colon as lesions 0-Is or 0-IIa (compare Sect. 10.7). In addition, several similar protruding lesions (0-Isp, 0-Is, 0-IIa) exhibit normal mucosal surface and submucosal vascular pattern such as submucosal tumor (SMT), rare hamartoma (Peutz-Jeghers polyp, juvenile polyp) or inverted diverticulum, which is soft and pliable. Reddish or isochrome polypoid or sessile lesions with normal or hyperplastic surface pattern are typical of inflammatory pseudopolyps in ulcerative colitis or Crohn’s disease (Fig. 10.4c, d; compare Chap. 11), rarely of sm-infiltrating lymphoma or secondary carcinoma originating from other sites or organs (peritoneum, ovary, metastatic cancer).
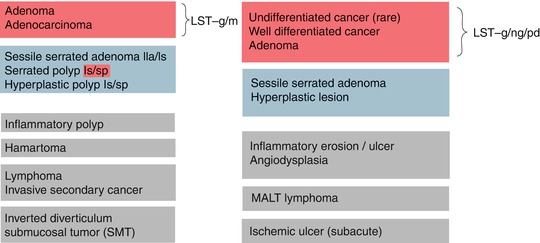
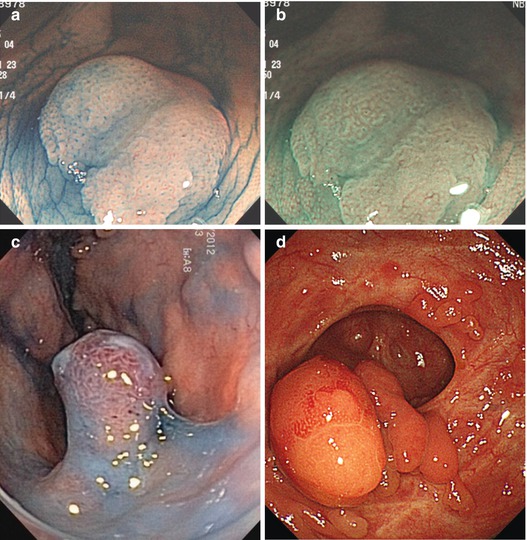

Fig. 10.3
Differential diagnosis of colorectal lesions according to pit pattern on indigo carmine CE: neoplastic (red), hyperplastic/serrated (blue), and normal pit pattern (grey). Mucosal neoplasias (adenomatous, serrated, and cancerous) exhibit distinct sharp margins, in contrast to hyperplastic or inflammatory lesions or diffuse submucosa-infiltrative neoplasias

Fig. 10.4
(a, b) Sessile hyperplastic polyp, PP II (stellar) in cecum, (a) indigo carmine CE; b) CP I (faint mesh) in cecum, M-NBI (40×). (c) Nonneoplastic 0-Ip (chronic inflammatory-regenerative lesion in moderately active ulcerative colitis), sigmoid colon indigo carmine CE. (d) Nonneoplastic 0-Isp and 0-Is lesion with unclear margin, in part visible CP I, PP II (inflammatory-regenerative, moderately active Crohn’s disease), sigmoid colon, WLI
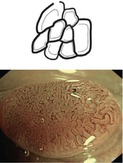
Flat or depressed lesions (0-IIa-c, often reddish) with key neoplastic signs (clear margins, neoplastic pit pattern, and disappearance of dendritic sm vascular pattern) are mucosal neoplasias. Reddish hyperemic lesions with uncertain margins comprise inflammatory mucosal lesions, such as erosions and inflammatory ulcer (often with fibrin coating, Fig. 10.5), ischemic ulcer, or angiodysplasia. Pale flat lesions with nearly normal pit pattern are typical for mucosal MALT lymphoma, or subacute ischemic ulcerations that show pale or mildly red lesions, but differ by bare proper muscle layer in the center surrounded by a margin of regular mucosa (lack of neoplastic pattern) (Fig. 10.6).
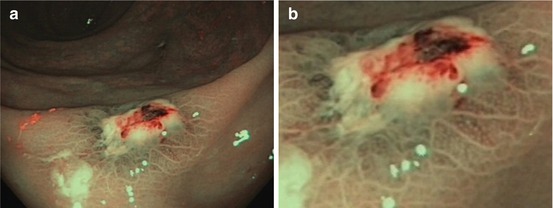
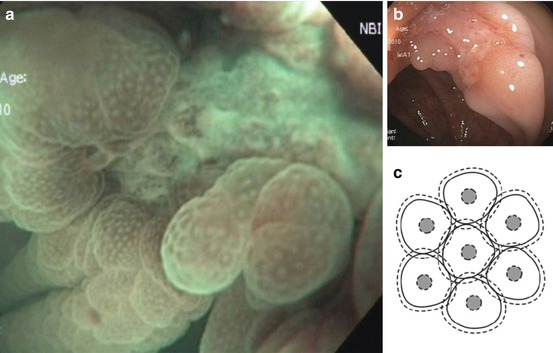

Fig. 10.5
(a) Solitary rectal ulcer in an 82-year-old man, standard NBI. (b) CP type I (meshed), PP type I, and uncertain margin of fibrin covered ulcer, standard NBI

Fig. 10.6
Lesion type 0-III. One of the two ulcers on neighboring haustral folds in the left transverse colon in an 80-year-old woman. (a) Standard WLI aspect. (b) Typical subacute ischemic ulcer with bare ground (proper muscle) and mucosal margins showing normal CP (meshed, CP I) and normal pit pattern (white dots, PP type I); M-NBI 80x. (c) Schematic of PP type I and CP type I (Modified from Sano et al. [17])
Pale flat lesions with disappearance of sm vascular pattern and some unclear margins are also compatible with LST-nongranular type, which however shows clear margins on magnifying NBI.
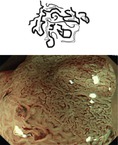
Flat and depressed neoplasias including LST-nongranular type and most LST-granular type – LST-GH, LST-GM, and LST-G-whole nodular – show discolored, often pale, areas with clear margins and disappearance of normal sm vascular pattern (Fig. 10.7a–j). They are further distinguished in classical adenoma, serrated adenomas or HNPCC-associated adenoma, and HGIN/intramucosal carcinoma (see IEE analysis, below). Depressed neoplasias type 0-IIc display air-induced deformation when infiltrating muscularis mucosae (MM) or superficial third of submucosa layer (sml) (Fig. 4.2b; compare below Sect. 10.6). Early cancer with invasion into sml often presents mild (0-IIc) or marked (IIc + IIa) surface depression (Table 4.2C).
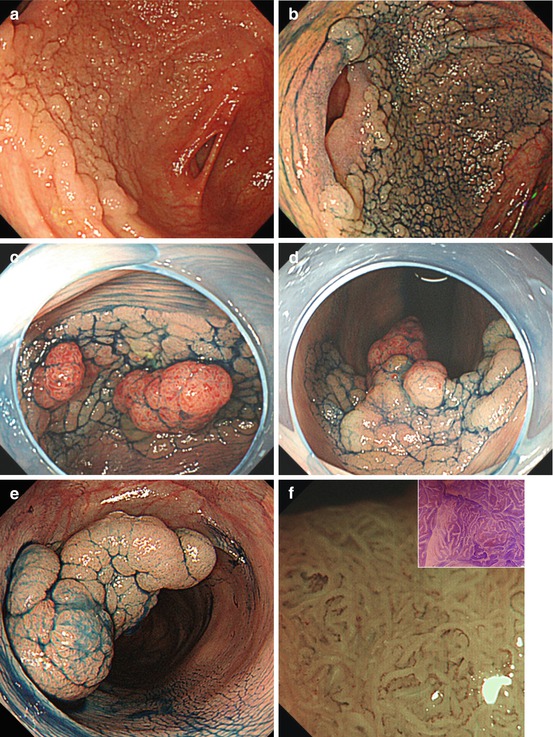
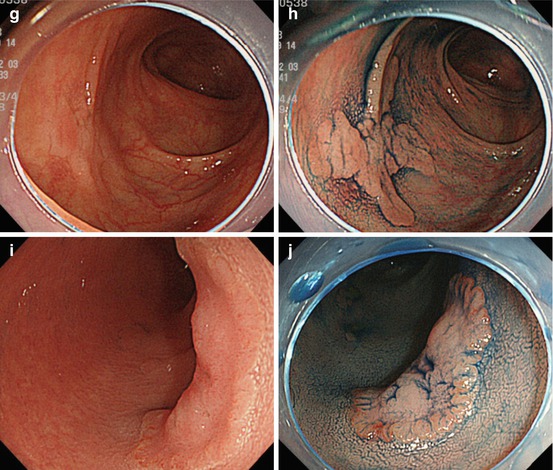


Fig. 10.7
LST-G. (a, b) LST-G granular homogenous type, cecum, (a) WLI; (b) indigo carmine CE. (c, d) LST-GM granular mixed nodular type, (c) WLI; (d) indigo carmine CE. (e) LST-G whole nodular. 0-Is + IIa, 30 mm in diameter, transverse colon, indigo carmine CE. (f) Same LST-GM as in (e) on M-NBI (80×): CP IIIA (insert with crystal violet CE: PP type VI low grade). ESD: tubular adenocarcinoma (intramucosal) LST-NG. (g, h) LST-NG flat (0-IIa); WLI and indigo carmine; (i, j) LST-NGPD (0-IIa + IIc, central protrusion), WLI and indigo carmine CE
Most LST-NG show normal color and relatively ill-defined margins; therefore, only larger size LST-NG are easily apparent on WLI endoscopy. Indigo carmine enhancement demonstrates distinct margins of the lesion (Fig. 10.7h, j). Prevalence of LST is highest in right colon as well as in rectum. Risk of focal cancer in different type LST is detailed in Table 10.3. The probability of malignant transformation of LST increases with size of the lesion, especially when >30 mm, and type, being very high in LST-GM, LST-NG, and highest in pseudodepressed LST-NGPD (Fig. 10.7i, j).
Mean | Percentage of lesion type | ||||||
|---|---|---|---|---|---|---|---|
Lesion | n | Size [mm] | LGIN (%) | HGIN (%) | Ca ≤sml (%) | Ca ≥sm2a (%) | |
 | LST-G(H) | 57 | 32 | 32 | 26 | 42 | 0 |
 | LST-G(M) | 86 | 39 | 9 | 30 | 56 | 5 |
 | LST-NG(F) | 77 | 22 | 26 | 34 | 36 | 3 |
 | LST-NG(PD) | 25 | 20 | 16 | 12 | 68 | 4 |
 | IIc and IIa + IIc | 6 | 17 | 0 | 0 | 33 | 67 |
Retrospective analysis (period 1998–2006) of LSTs (≥20 mm size) resected at the National Cancer Center, Tokyo, confirmed sm-invasive cancer in 0.9 % of LST-GH, 16 % of LST-GM, and 58 % of LST-NG but only in 5 % of small size (d < 20 mm) LST-GM or LST-NG. Hence, NCC recommends resection en bloc for LST-NG of size ≥20 mm and LST-GM ≥40 mm [18].
10.5 Differential Diagnosis of Colorectal Lesions Using Magnifying Image-Enhanced Endoscopy
Image-enhanced magnification endoscopy (IEE) using NBI for (CP) indigo carmine for PP II-IV and crystal violet CE for PP V is basic for accurate (>90 %) endoscopic differential diagnosis of early mucosal neoplasias, in order to predict histologic type of neoplasia and tumor category (algorithm see Fig. 10.8). First, CP is diagnosed on M-NBI, then decision on indigo carmine or crystal violet CE is made for diagnosis of PP.

Fig. 10.8
Analysis of colorectal mucosal neoplasias with magnifying NBI/chromoendoscopy, to distinguish malignancy and grade of invasiveness by capillary pattern (CP) and pit pattern (PP). § PP type V I high grade with encroachment of margins signals deep sm invasion. *superficial sm invasion <1,000 μm; **deep sm invasion ≥1,000 μm
Note
IEE with magnification (≥60×), NBI, and often crystal violet CE is required to accurately (>90 %) differentiate by CP and PP:
Adenoma versus carcinoma
Intramucosal versus submucosa deeply invasive carcinoma
Hyperplastic lesion versus adenoma and serrated neoplasias
(the latter distinction is less accurate; compare Sect. 10.6)
Hyperplastic lesions type 0-I or IIa are mainly hyperplastic polyps (HP) and are frequently seen in rectosigmoid colon (Fig. 10.4). Sporadic hyperplastic polyps are nonneoplastic displaying stellar PP type II (Fig. 10.2b) and scanty, regular CP type I (Table 10.2a; Fig. 10.4).
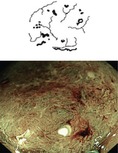
Adenomas consist of transformed colonocytes with enhanced nucleus/cytoplasm ratio, loss of polar orientation of cell nuclei in the epithelial layer, enhanced clonal proliferation of colonocytes, and formation of pseudoglandular structures (compare Fig. 4.6). By definition, adenomas lack invasive or metastatic potential, and the process of cell-cell adhesion is preserved. Therefore, the lesion forms single layered, glandular marginal epithelium, seen as surface pattern (SP) using NBI with magnification (Fig. 4.5). The enhanced proliferation of pseudoglandular structures creates patterns of different surface shapes. Adenomas typically show regular pseudoglandular structure, visualized as PP type III l or IV, rarely IIIs or Viregular (Fig. 10.2c–g), and dense CP type II (Figs. 10.9 and 10.10). The margin of adenoma is clearly visible on WLI (and NBI) by change of type in surface pattern but without demarcation of surface relief (Fig. 10.9d, compare Fig. 4.6). The regular structure of adenomatous epithelium is well visualized by absorptive staining of colonocytes using crystal violet. Crystal violet best demonstrates irregular or destroyed pseudoglandular structure (PP V I or V N ) (Fig. 10.2f–h).
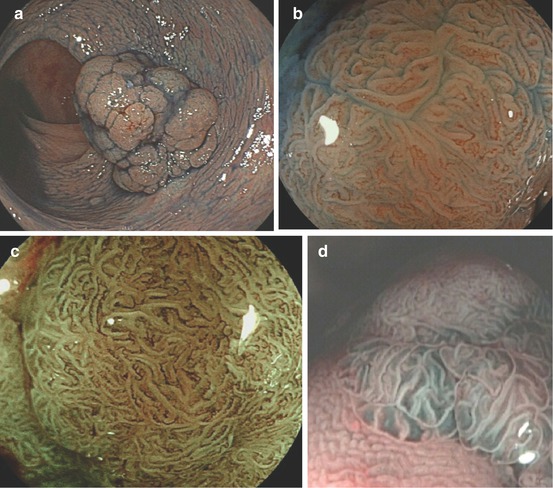
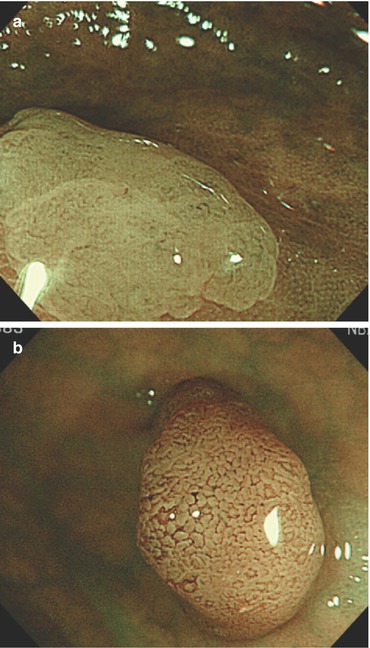
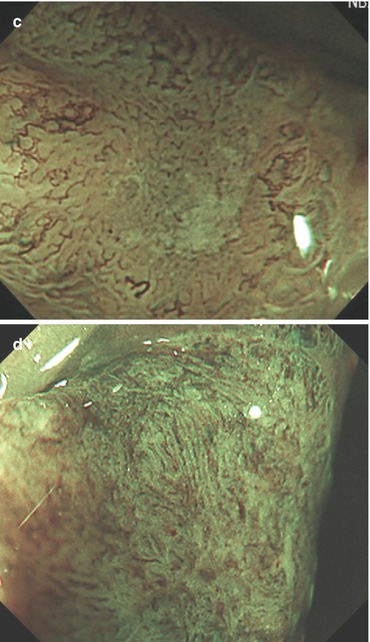

Fig 10.9
(a–d) Protruding neoplasia 0-Isp, 25 mm in diameter, (b) WLI indigo carmine CE (c) with magnification, (d) M-NBI (80-fold): PP type IV and CP type II. Histology: tubulovillous adenoma with focal HGIN. (d) Protruding adenoma 0-Isp, 15 mm in size, clear margin without demarcation of relief, CP type II (and PP type IIIl), even surface pattern (SP marginal crypt epithelium; M-NBI 60-fold). EMR tubular adenoma with LGIN


Fig. 10.10
Capillary pattern (CP) types (M-NBI, 100×). (a) CP type I meshed is faintly visible (−) in hyperplastic lesion type IIa (with PP type II), as compared to CP type I (+), visible in adjacent normal mucosa (right side). (b) CP type II, regularly meshed, in lesion 0-Is is typical for adenoma (probable PP IIIl) (c) CP type IIIA, irregularly meshed, dense capillary pattern, in flat lesion compatible with adenoma and HGIN or intramucosal (or superficially sm-invasive) differentiated cancer. Crystal violet CE is recommended for evaluation of pit pattern. (d) CP type IIIB, loosely irregular, and in part sparse capillary vessels suggesting sm-invasive early cancer (≥sm2). Crystal violet CE is required to categorize the corresponding pit pattern type V (e.g., high-grade irregular or nonstructured)
Note
Adenoma shows typical structural findings on WLI and indigo carmine:
Disappearance of submucosal vascular pattern
Clear lateral margins of the lesion
Reddish in color, lobulation on the lesion surface
Regular pit pattern, tubular (III l sometimes IIIs) or branched (IV)
Even distension of type 0-IIa + IIc adenomas on insufflation/desufflation, and
Typical structural findings on magnifying NBI:
Even surface pattern (marginal crypt epithelium)
Regular network microvascular pattern (CP type II)
Differentiated adenocarcinoma (G1, G2) exhibits irregularities in thickness and shape of cancerous marginal crypt cell layers (irregular SP) and irregular pseudogland structure (irregular pit pattern PP type V I or V N on crystal violet CE) (Figs. 10.8 and 10.2f–h, compare Sect. 10.9, case no. 1). Angiogenesis created irregular dense capillary pattern CP type IIIA [10, 12, 19, 20] (Figs. 4.4b, 10.7f and 10.10c). Coherently growing cancer cell clusters exhibit sharp margins with “demarcation line” of surface relief toward surrounding adenomatous or normal epithelium. Deep sm-invasive cancer destroys, at least in part, pseudogland structure and microcapillaries and creates destructive, amorphous pit pattern (PP V I high grade, V N ) and irregular, sparse microvessels with varying thick caliber (CP type IIIB) (Figs. 10.2g–h and 10.10d).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








