- Colorectal cancer is the third leading cause of cancer death for men and women combined, and incidences and mortalities are higher in individuals older than 50 years. A sedentary lifestyle and regular high intake of red meat maybe associated with increased risks of developing colorectal cancer (CRC).
- Patients diagnosed with ulcerative colitis have higher risk of developing CRC than those without known risk factors. Almost all individuals diagnosed with familial adenomatous polyposis will develop CRC without a prophylactic colectomy by age 40.
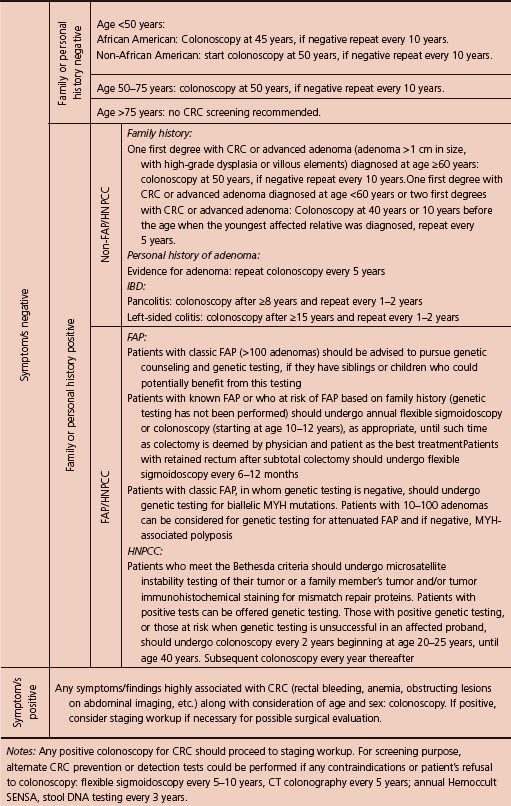
- Early detection is lifesaving. Every person with no family history of CRC should be screened with colonoscopy at the age of 50 years and 10 years earlier if there is a positive family history.
- Endoscopic ultrasound is the gold standard to stage rectal cancer.
- Neoadjuvant chemoradiation for stages II and III rectal cancer demonstrates better outcomes on disease-free recurrence.
- For patients undergoing curative resection of CRC, overall survival rates vary between 55% and 75%, with most recurrences seen in the first 2 years of follow-up.
- Surgical resection should be considered for resectable isolated liver or pulmonary metastases.
- Fluoropyrimidine is still the mainstay chemotherapeutic agent. Addition of cetuximab to the conventional chemotherapeutics shows favorable outcomes for K-RAS wild-type patients, across all lines of therapy.
- Monitoring carcinoembryonic antigen level is recommended during treatment for evaluation of disease recurrence or metastasis.
Provides accurate, up-to-date, comprehensive cancer information from the US government’s principal agency for cancer research.
American Cancer Society—Supports research, patient services, early detection, treatment, and education.
American Society of Clinical Oncology—Provides practice guidelines, research resources, education and training, and public policy.
National Comprehensive Cancer Network—Provides clinical practice guidelines on oncology.
CDC (Centers for Disease Control and Prevention)—Promotes colorectal (colon) prevention by building partnerships, encouraging screening, supporting education and training, and conducting surveillance.
Surveillance Epidemiology and End Results by National Cancer Institute (NCI)—Provides information on cancer statistics.
- The potential negative physical and psychological effects associated with a false-positive colonoscopy include anxiety induced by fear of being diagnosed with colorectal cancer.
- Permanent stoma is a major quality-of-life concern in the management of colorectal cancer postoperatively.
- Pre- or postoperative radiation may result in genitourinary and sexual dysfunction.
- Providing the appropriate remedy for chronic malignant pain is the most important challenge in managing the end-stage colorectal cancer patient.
- Finally, colorectal cancer requires multidisciplinary interaction between gastrointestinal medical oncology, surgical oncology, radiation oncology, and pathology to execute a sustainable and favorable outcome.
Introduction
The diagnosis and treatment of cancer has a long history stretching back to early human civilization. Hippocrates (460–370 BC), the father of medicine, first used the word “Karkinos” (the Greek word for crab) to describe the difference between benign and malignant tumors.5 Ancient Chinese civilizations used special herbs to treat the symptoms associated with colorectal cancer (CRC) for decades.6
CRC remains one of the most challenging malignancies to treat. Historically, CRC care has always been costly. The US national cost of CRC care in 2010 (including the initial care year after diagnosis, continuing care, and last year of life care) was $14.14 billion.7
Breakdown of CRC care cost:
- Risk assessment
- Primary prevention
- Screening/detection
- Diagnosis
- Treatment
- Recurrence surveillance
- End-of-life care
According to the American Cancer Society’s “Colorectal Cancer Facts and Figures 2011–2013,” CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death in both men and women. Globally, more than 1.2 million new cases and 608,700 deaths occurred in 2008.8 According to the American Cancer Society (ACS), 141,210 people were diagnosed with CRC and 49,380 people died of the disease in the United States in 2011.
From 2003 to 2007, the median ages at diagnosis and death of CRC were 70 and 75 years of age, respectively.9 According to the SEER (Surveillance, Epidemiology, and End Results) database, lifetime risk in men for developing CRC is 1 in 19 (5.2%) and in women is 1 in 20 (5.0%).
Incidence
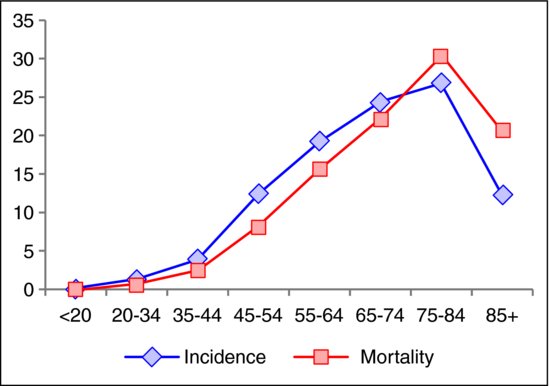
In general, about 90% of new cases occur in individuals 50 years and older (15 times more than in age between 20 and 49, according to SEER), and the incidence is 35% higher in men than in women and racially highest in African Americans.10 CRC incidence rates have been declining in the United States since the 1980s.9 Since 1998, rates have been declining by 3.0% per year in men and by 2.3% per year in women.11 Moreover, for that period of time, rates have declined among men and women in every major racial/ethnic group except for American Indian/Alaskan Native women (Figure 5.2).11 But CRC incidence rates are rapidly increasing in Spain and in a number of Eastern Asian and Eastern European counties.12,13 Table 5.1 shows the percent of US men and women developing CRC over 10- and 20-year intervals.
Table 5.1 Percent of US men and women who develop colorectal cancer over 10- and 20-year intervals according to their current age during 2005–2007.
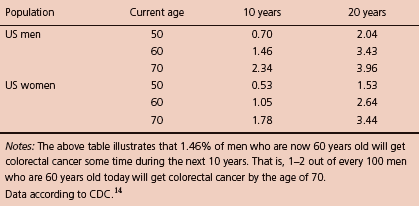
Figure 5.1 Colorectal cancer mortality and incidence in different age groups from 2003 to 2007 in the United States. (Data according to SEER.9)
Mortality
About 94% of deaths from CRC occur in individuals 50 years and older, and the mortality is 40% higher in men than in women and racially highest in African Americans.10 Figure 5.1 shows the mortality and incidence graph of CRC for different age groups. Since 1998, the overall mortality rates in the United States have decreased by 2.8% per year in men and 2.6% per year in women, and have been generally decreasing in both sexes in every major racial/ethnic group except for American Indian/Alaska Native men and women (Figure 5.2).11
Figure 5.2 Colorectal cancer mortality and incidence trends from 1997 to 2006 in the United States. (Data according to Center for Disease Control (CDC).14)
Research funding
CRC is an active area of scientific research. The National Cancer Institute’s (NCI) reports show a gradual increase in the US national CRC research funding since 2006 (FY 2006: $244.1 million, FY 2007: $258.4 million, FY 2008: $273.7 million, and FY 2009: $308.6 million (including funding from The American Recovery and Reinvestment Act 2009 signed by President Obama, and funding by ACS for more than $80 million)10).
Cancer distribution
- Cecum: 16%.
- Ascending colon: 7%.
- Hepatic flexure: 3%.
- Transverse colon: 6%.
- Splenic flexure: 2%.
- Descending colon: 4%.
- Sigmoid colon: 31%.
- Rectum: 31%.
The development of CRC depends on two distinct pathways, both characterized by genetic instability: microsatellite instability and chromosome instability.15 The first pathway involves oncogenes (KRAS, BRAF, MYC, etc.) and tumor-suppressor genes (TP53, APC, etc.), which directly regulate cell birth and cell death.16 In the second pathway, stability genes (hMSH26, hMLH1, hPMS2, etc.) are involved that do not directly regulate cell birth or death but rather control the mutation rate of other genes, including growth controlling genes.
Molecular types17
CIMP, CpG island methylated phenotype; MSS, microsatellite stable; MSI, microsatellite instable:
Nonmodifiable risk factors
Age and hereditary factors are the most important nonmodifiable risk factors.
The lifetime risk of developing colorectal adenomas is nearly 19% in the US population and nearly 95% of sporadic CRCs develop from these adenomas.18 After histological type, size and duration are the next important indicators of risk of developing CRC. A polyp of 1 cm in diameter has a one in six (17%) chance of developing into a cancer over 10 years.19 For already cancerous polyps, the most important prognostic factor is the depth of invasion.
Genetic patterns of CRC
- Sporadic: 60–85%.
- Familial: 10–30%.
- Hereditary nonpolyposis colon cancer (HNPCC): 5%.
- Familial adenomatous polyposis (FAP): 1%.
- Autosomal dominant inheritance other than FAP and HNPCC: <1%.
FAP
FAP is an autosomal dominant disorder of APC gene mutations that affects 1 in 1000 births,19 characterized by hundreds to thousands of polyps (Figure 5.3) arising within the colorectal region. Each polyp has the potential to become malignant as early as age 20 and by age 40; almost all people with this disorder will develop CRC without a prophylactic colectomy. About 25% of FAP patients do not have positive family history, and they should be tested for biallelic MYH mutation.20
Subtypes of FAP
- Gardner’s syndrome: polyposis, epidermoid cysts, osteoma.
- Turcot’s syndrome: polyposis, CNS tumors (medulloblastoma).
- Other extracolonic manifestations: dental abnormalities, congenital hypertrophy of the retinal pigmented epithelium (CHRPE), pancreatic and thyroid tumors, etc.
HNPCC/lynch syndrome
HNPCC is the most common inherited type of CRC. It is an autosomal dominant disorder caused by a mutation in one of the mismatch repair genes (hMLH1, hMSH2, hMSH6, or PMS2) that are responsible for proofreading DNA during replication and thus maintaining genomic stability.19 HNPCC is associated with an 80% lifetime risk of CRC with the majority occurring on the right side of the colon.19
Amsterdam criteria II for diagnosis of HNPCC
- Three or more family members with HNPCC-related cancers, one of whom is a first-degree relative of the other two.
- Two successive affected generations.
- One or more of the HNPCC-related cancers diagnosed under age 50 years.
- FAP has been excluded.
MYH-associated polyposis
MYH-associated polyposis (MAP) is an autosomal recessive inherited disease. Mutations of the MYH gene (caretaker) are thought to lead to somatic mutation of APC (gatekeeper).20 Usually the patients do not have a positive family history. There is a twofold increase in the incidence of CRC among patients with MAP, compared with the general population.22 Most patients are ≥45 years of age at the time of diagnosis, with 10–100 polyps.20
Peutz–Jeghers syndrome
Peutz–Jeghers syndrome (PJS) is an autosomal dominant disease that presents with hamartomatous polyps and mucocutaneous melanin pigmentations. Lifetime risk for CRC development is 39%.20 Currently only mutations in STK11 (LKB1) have been identified as cause for PJS.
Juvenile polyposis syndrome
Juvenile polyposis syndrome (JPS) is an autosomal dominant disease that presents with hamartomatous polyps. Lifetime risk for developing CRC is 20–60% and the median age of diagnosis is 35–40 years.20 The genes known to be associated with JPS are BMPR1A and SMAD4.
Inflammatory bowel disease
First portrayed by both Crohn and Rosenberg in 1925 in a report,23,24 the increased risk of CRC in patients with ulcerative colitis (UC) and Crohn’s disease (CD) has long been recognized.18,19,23,25 The risk of CRC with associated UC is twofold higher26,27 compared with the normal population, and CRC is observed in 5.5–13.5% of all patients with UC and 0.4–0.8% of patients with CD.26 Based on long-term follow-up in a subset of studies included in the meta-analysis, Eaden’s group estimated that the cumulative risk of CRC in UC patients to be 1.6% at 10 years, 8.3% at 20 years, and 18.4% at 30 years. Lesions are often found proximal to the splenic flexure and are of mucinous type.27 The pathology of developing CRC in inflammatory bowel disease (IBD) patients begins with no dysplasia, progressing to indefinite dysplasia, low-grade dysplasia (10% risk of CRC20), high-grade dysplasia (30–40% risk of CRC20), and finally to carcinoma. The incidence of KRAS mutations has been found to be reduced in UC-associated CRC.28–30 BRAF mutations have been reported in 9% of IBD-associated CRC.28,31
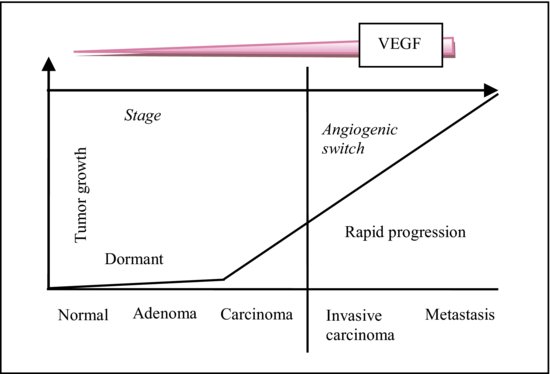
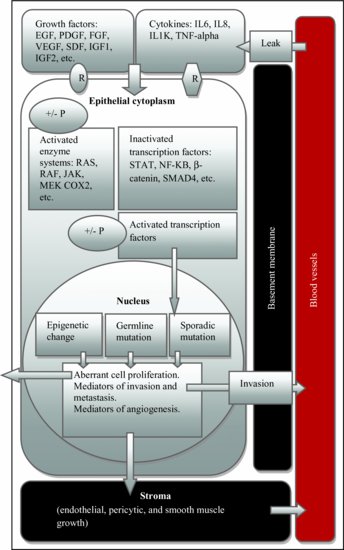
The tumor microenvironment includes cells (fibroblasts, endothelia cells, and infiltrating immune cells), molecules, and blood vessels that surround and feed a tumor cell.31 Figure 5.4 shows the progressive increase of vascular endothelial growth factor (VEGF) that effects the growth of CRC. Figure 5.5 depicts how tumor microenvironment interplays in the development of CRC.
Figure 5.5 Microenvironment and colorectal cancer development. EGF, endodermal growth factor; PDGF, platelet-derived growth factor; FGF, fibroblast growth factor; VEGF, vascular endothelial growth factor; SDF, stromal cell-derived factor; IGF, insulin-like growth factor; TNF, tumor necrosis factor; STAT, signal transducer and activator of transcription; IL, interleukin; +/− P, phosphorylation or dephosphorylation; R, receptor.
Metabolic environment and neoangiogenesis
Due to intermittent blood flow in a tumor and resultant hypoxia, there is a propensity for increased free radical production. Reperfusion injury may also apply additional selection pressure on cancer cells.35 Hypoxia and low pH alter the regulation of various angiogenic growth factors including VEGF, Ang2, PDGF, TGF, COX1-2, iNOS, endothelin-1, -2, HGF, IL-8, tissue factor, PAI1, and thrombospondin-1, -2.35,36
Epithelial–mesenchymal transition
Cancer cell detachment from the primary site is one of the key initial events required for metastasis to occur. It is closely associated with epithelial–mesenchymal transition (EMT), which is a morphogenetic process where epithelial cells lose their characteristics and gain mesenchymal properties during embryogenesis.37 One of the major elements that characterizes EMT of carcinoma cells is the loss of E-cadherin-mediated cell–cell adhesion.38,39
The Wnt signaling pathway plays a key role during embryonic development43 as well as in CRC development.32 The Wnt families of proteins are involved in cell–cell signaling and adhesion during embryonic development. This includes the formation of the embryonic axes to end-stage organ development by regulation of β-catenin intracellular location and function.32 Lack of regulation of the Wnt/β-catenin pathway is observed in CRC.32
The morphologic adenocarcinoma sequence was recognized at least 40 years ago and several studies, including those of Vogelstein’s group (Figure 5.6) and National Polyp Study, suggest that CRC results from sequential accumulation of genetic and molecular alterations.44 Although the earliest identifiable lesion in CRC formation is the aberrant crypt focus,45,46 there are currently two proposed morphological pathways of spontaneous microadenoma development. First is the “bottom-up” theory where a stem cell situated in a niche in the crypt base acquires a second mutation and subsequently expands stochastically, producing neoplastic daughter cells. These cells migrate upward to colonize the entire crypt and form a clonal monocryptal adenoma that eventually further replicates and undergoes crypt fission.47 Second is the “top-down” theory where an initial stem cell mutation occurs in the epithelial mucosa located between two crypt orifices with subsequent stem cell division producing a mutant clone that expands laterally and downward into the crypt displacing the normal epithelial cells.47 Based on early investigations of non-FAP adenomas, about 50% of samples showed LOH for APC in the upper portion of the crypts with nuclear localization of β-catenin, verifying the loss of a gene in the Wnt pathway, most likely APC.47 Ultimately, the product of the APC-Crypt fission sequence, along with other gene alterations (as outlined in Figure 5.6), acquires malignant characteristics, penetrates the muscular layer that lies underneath the lamina propria, and becomes an invasive carcinoma.45,46
Figure 5.6 Vogelstein’s model. (Photos by Chris Macklin, Consultant Colorectal Surgeon, Mid Yorkshire Hospitals NHS Trust, UK.)
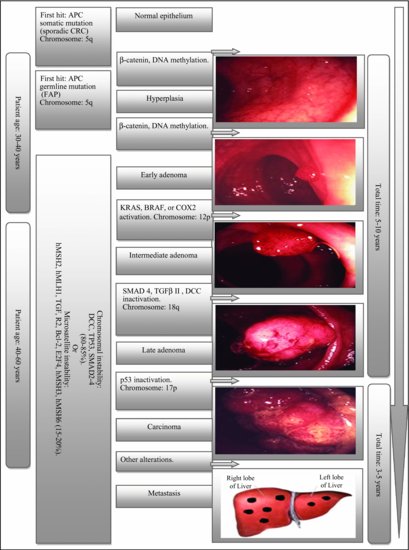
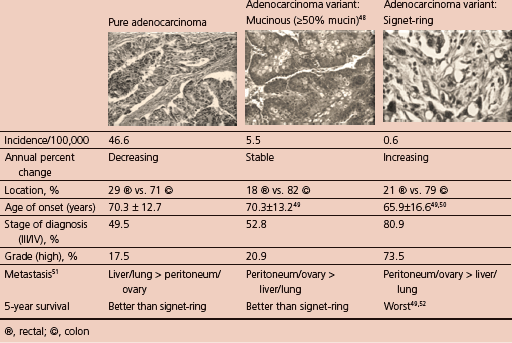
Histological variants of colorectal cancer
Pure adenocarcinoma is mostly occurred in histological variant of CRC followed by mucinous and signet-ring type (Table 5.2).
Table 5.2 Major histological variants of CRC.
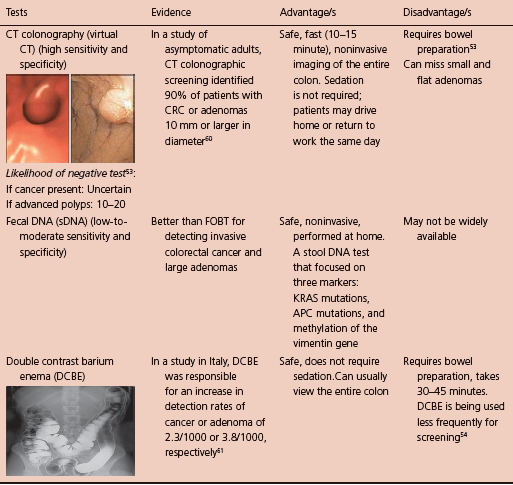
Screening tests are necessary to detect the predisease conditions that lack clinical features (primary prevention). Screening tests can also find early stage colon cancer (secondary prevention).14,53 The cost-effectiveness of CRC screening is estimated to be approximately $40,000 per year of life gained.54 Unfortunately, less than half of Americans are up to date in terms of CRC screening.55
The American College of Gastroenterology (ACG) continues to recommend that screening begins at the age of 50 years in persons with average risk (without a family history of CRC, personal history of adenomas, or familial syndromes associated with CRC), except for African Americans who should begin at the age of 45 years.56,57 The current evidence also supports a decision by clinicians with individual patients possessing an extreme smoking history or obesity to begin screening at an age earlier than 50 years and perhaps as early as 45 years.57
According to the AGC, the screening tests are subgrouped into (A) cancer prevention tests and (B) cancer detection tests.58
Cancer prevention tests: These tests have the potential to image both cancer and polyps. Colonoscopy is the gold standard.57 Double contrast barium enema, flexible sigmoidoscopy, and CT colonography (virtual CT) are also under this category.59
Cancer detection tests: These tests have a low sensitivity for polyps and typically a lower sensitivity for cancer. Annual fecal immunochemical test to detect occult bleeding is the test of choice.57 Fecal occult blood test and stool DNA test are also in this category.59 Colonoscopy should be done if any of these are positive.
Physical
Digital rectal exam
Less than 10% of CRCs are located within the 7–8 cm reach of the examining finger. Stool obtained during a digital rectal exam (DRE) is inadequate to screen for the presence of blood, and there is no evidence that DRE reduces mortality from CRC and thus it is not indicated as a screening test for the prevention or early detection of CRC.54
Available screening tests are discussed in detail in Table 5.3. This table has been largely based on “The CRICO/RMF Colorectal Screening Algorithm” publication.56
Table 5.3 Available screening tests.

A CRC screening and diagnosis algorithm is outlined in Table 5.4 (based on CRICO/RMF, ACG, and US Preventive Services Task Force (USPSTF)).
Table 5.4 CRC screening and diagnostic algorithm.56,57,62
Imaging
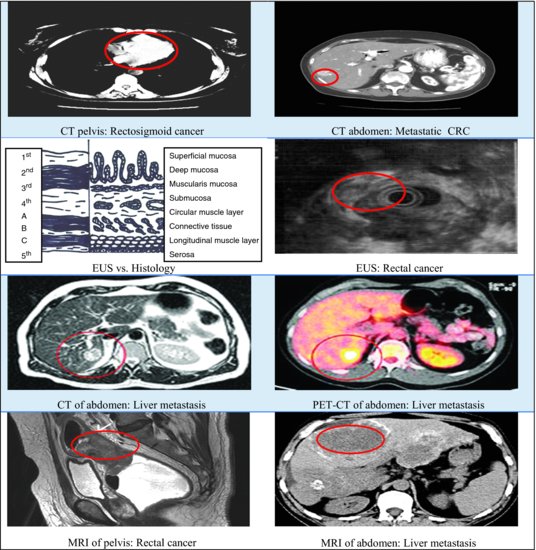
Imaging is vital in CRC management. Not only is it important for diagnosis and guiding biopsies but it is also crucial in assessing extent of disease progression and thus determining treatment.63 Figure 5.7 describes the imaging studies available to stage CRC.
X-ray
The disadvantage of this technique is that all structures are projected in one plane, and although there is good contrast between air, soft tissue, and bone, the inherent soft-tissue contrast is too low to allow differentiation of various soft-tissue structures (e.g., a tumor in muscle or a metastasis in the liver).63 Given that conventional x-ray images show limited contrast, they are only of value if the tumor absorbs substantially more or less of the x-ray beam than surrounding tissues, consequently distinguishing tumor from surrounding tissue is only possible in lung and bone cancer, not in CRC.63
CT
CT imaging has good spatial and contrast resolution: all tissues can be visualized three dimensionally in an investigator-independent and highly reproducible way.63 Although earlier studies stated the accuracy ranging between 85% and 90%, larger studies showed that the accuracy was more in 50–70% range, varying directly with the stage of the tumor.64 Results from a multi-institutional study reported 74% accuracy of CT assessment of wall invasion and a sensitivity of 48% in evaluating lymph node metastases. CT demonstrated 85% accuracy and 97% specificity in detecting liver metastases.65
MRI
Advantages of MRI include the combination of high resolution and high soft-tissue contrast, the possibility to obtain direct images in any plane, and the potential for enhancing specific imaging characteristics by the use of MR-contrast agents.63 The visibility of these contrast agents is 1000-fold higher than with iodine x-ray contrast agents that in turn enhance the tumor visibility considerably.63 Disadvantages of MRI are (1) long examination time, significantly longer than a CT scan and (2) lack of uniform technique, though by using general protocols and the same type of MR scanner, reproducibility can be achieved.63 MRI has revolutionized the management of more advanced rectal cancer. With the use of external coils, the overall accuracy of MRI for the T staging (depth of tumor) of rectal cancer varies from 65% to 86%, with considerable interobserver variability (recent studies reported 86–100%).66 Nodal metastases (N staging) were better depicted.66,67 In meta-analysis of pooled sensitivity and specificity of MRI for predicting circumferential margin involvement, they were reported to be 94% and 85%, respectively.68
EUS
Endoscopic ultrasound (EUS) has become the gold standard procedure for staging rectal carcinoma.69–71 EUS enables one to distinguish layers within the rectal wall72; it appears to be an accurate method for detecting depth of tumor penetration and perirectal spread.73,74 The accuracy of T staging for rectal carcinoma has been reported up to 80–96%.64,72 The assessment of nodal involvement is less accurate than T staging with accuracies around 75%.72,75 The major disadvantages are (1) it is unable to outline the M staging and (2) it tends to overstage cancers.72,75
Indications for EUS in rectal cancer75
- Large cancers: to determine candidacy for preoperative radiation/chemotherapy.
- Small cancers: to determine candidacy for transanal excision.
Potential impact of EUS staging on rectal cancer management75
- uT1: transanal local resection.
- uT2: radical resection ± postoperative radiation.
- uT3–4 or uN1: preoperative chemoradiation before radical resection.
FDG-PET
FDG is a glucose analog and its uptake reflects cellular metabolism, which is increased in many tumors.63 But it is also accumulated in activated inflammatory cells such as granulocytes and macrophages, thus making the differentiation between tumor and normal tissue response difficult.63 The lack of anatomic detail has been solved by the introduction of integrated PET-CT scanners.63 Compared with CT staging alone, PET-CT is significantly more accurate in defining TNM stage (difference 22%; 95% CI 9–36%; p = 0.003),76 and has been shown to yield a cost savings of $2761 per patient,64 and to avoid exploratory surgery in 6.1% of patients.
The treatment intent for CRC could be curative or noncurative (palliative, symptomatic relief, etc.). The available modalities are surgery, chemotherapy, radiotherapy, and chemoradiotherapy (Table 5.5).
Table 5.5 Treatment options for CRC.77
| Stage | Colon | Rectal |
| Stage I T1-2N0M0 | Definitive surgical resection | Same as colon |
| Stage II T3-4bN0M0 | Definitive surgical resection ± adjuvant chemotherapy | Preoperative chemoradiation + definitive surgical resection + adjuvant chemotherapy |
| Stage III any T, N1-N2a-b, M0 | Definitive surgical resection + adjuvant chemotherapy | Preoperative combined chemoradiation + definitive surgical resection + adjuvant chemotherapy |
| Stage IV M1a-b with any T or N | Preoperative chemotherapy ± radiation therapy ± palliative/definitive primary vs. metastatic surgical resection | Same as colon |
| AJCC7: T4a, penetrates through the surface of the visceral peritoneum; T4b, directly invades or adheres to other organs or structures; N2a, 4–6 nodes; N2b, >6 nodes; M1a, confined to one organ or sites; M1b, to >1 organs/site or to the peritoneum. | ||
Surgery is the only cure for CRC. Eighty percent of patients present without detectable metastases. For colon cancer, surgery is usually the first step, whereas for rectal cancer, a preoperative MRI or EUS is required to determine whether the patient clinically has stage II or III disease, in which case neoadjuvant chemoradiation is offered off study.78
The principles of surgery for CRC include en bloc resection to achieve a complete resection with negative margins, lymphadenectomy to the level of the primary feeding vessel, and removal of at least 12 regional lymph nodes.79 By definition, regional lymph nodes include those nodes along the vascular arcades of the marginal artery and those adjacent to the colon/rectum along its mesocolic/rectal (upper third) border. Involved nodes outside the primary node-draining basin and extraregional lymph nodes are staged as metastatic disease.80 During surgery, the abdomen should be explored to rule out metastasis to the peritoneum, distant lymph nodes, or other visceral organs. Approximately 10% of CRC patients have invasion of adjacent organs or inflammatory adhesions involving neighboring structures. According to the guidelines of the National Comprehensive Cancer Network (NCCN), NCI, and the American Society of Colon and Rectal Surgeons, the appropriate surgical management for CRCs that grow through the bowel wall into adjacent structures or organs should include multivisceral resection with a negative margin of the adjacent structure(s).80 Data suggests that laparoscopic colectomy can be a safe81 alternative to open colectomy without compromising long-term outcomes.82,83 For rectal cancer, a wide anatomical resection of the mesorectum with ideal bowel margins at least 2 cm distally and 5 cm proximally, as well as clear radial margins, is optimal.79 Concerns about achieving negative lateral and circumferential margins have been addressed by the wider acceptance of total mesorectal excision for rectal cancers. The mesorectum is the initial site of spread for rectal cancer, making its complete removal in a single anatomic block an important feature of rectal surgery.84
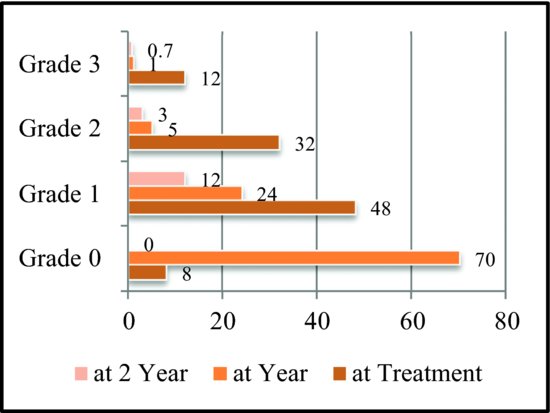
For patients undergoing curative resection of CRC, overall survival (OS) rates vary between 55% and 75%, with most recurrences seen in the first 2 years of follow-up.85 For node-negative patients, survival with surgery alone mimics that of general population’s.86 Surgery is also curative in 25–40% of highly selected patients who develop resectable metastases in the liver and lung.87,88
Procedures
For colon cancer80:
- Right hemicolectomy: for tumors of the cecum, ascending colon, and hepatic flexure.
- Extended right hemicolectomy: for tumors of proximal, mid, and distal transverse colon.
- Transverse colectomy: for tumors of mid transverse colon.
- Left hemicolectomy: for tumors of distal transverse, descending, and proximal sigmoid colon.
- Sigmoid colectomy: for tumors of sigmoid colon.
- Subtotal and total colectomy: for synchronous tumors of right and left side.
For rectal cancer:
- Transanal excision: for early, small, and clinically lymph node negative tumor.
- LAR: for proximal and mid rectal tumor.
- Abdominoperineal resection: for distal rectal tumors. Permanent colostomy needed as anal sphincter cannot be preserved.
Sentinel node mapping
According to the sentinel lymph node (SLN) hypothesis, tumor cells migrating from a primary tumor colonize one or a few lymph nodes (first few in the chain) before involving other lymph nodes.80 Injection of vital blue dye with or without radiolabeled colloid around the area of the tumor permits identification of an SLN in the majority of the patients, and its status accurately predicts the status of the remaining regional lymph nodes. It has been established that SLN mapping has altered the surgical management of breast cancer; however, this is not the case in CRC. SLN mapping carries a false-negative rate of approximately 10% in larger studies, but will also potentially upstage a proportion of patients from node negative to positive following the detection of micrometastases; hence, the prognostic implication of these micrometastases requires further evaluation.89
Medical therapy
The role of medical treatment is usually as adjuvant to the definitive treatment of primary and resectable metastases, or as palliative in case of metastatic disease. In general, adjuvant chemo is not indicated for all stage II CRC90–92; this is according to the Cancer Care Ontario Program in Evidence-Based Care, an expert panel convened by the American Society of Clinical Oncology (ASCO) and the NCCN.84 Pooled analyses and meta-analyses have shown a 2–4% improvement in OS for patients with adjuvant single agent fluorouracil (5-FU) based therapy compared with observation.87,93–95 But it may be considered for high-risk stage II that includes any of the following84,90 (also according to the study ECOG INT-0035 (Table 5.6)):
Table 5.6 Key adjuvant clinical trials.

- T4 primary tumor.
- Poorly differentiated histology.
- Lymphovascular invasion.
- Perineural invasion.
- Bowel obstruction or perforation.
- Less than 12 regional lymph nodes in the surgical specimen.
- Positive surgical margins.
- MSS.
Commonly used combination chemotherapy regimens are discussed in Table 5.7.
Table 5.7 Available regimens.
| Regimen | Description |
| Roswell Park (NSABP) | 5-FU + leucovorin, IV; cycle every 8 weeks for 4 cycles. No longer in use |
| Mayo Clinic (NCCTG) | 5-FU + leucovorin, IV; cycle every 4 weeks for 6 cycles. No longer in use |
| LV25FU (de Gramont) | High-dose LV as a 2-h infusion followed by 5-FU as an intravenous bolus plus a 22-h continuous infusion, 2 days for 2 weeks |
| FOLFIRI (Doulliard) | 5-FU+ leucovorin + irinotecan, IV; cycle every 14 days |
| FOLFOX 4, 6, 7 | 5-FU + leucovorin + oxaliplatin, IV; cycle every 14 days |
| FOLFOXIRI | 5-FU + leucovorin + oxaliplatin + irinotecan, IV; cycle every 14 days |
| IFL (Saltz) | Irinotecan + 5-FU + leucovorin, IV: cycle every 6 weeks |
| Capecitabine | PO; cycle every 21 days |
| CAPIRI | Capecitabine + irinotecan; cycle every 21 days |
| CAPOX | Capecitabine + oxaliplatin; cycle every 21 days |
| Irinotecan | Cycle every 6 weeks or 21 days |
p = 0.023).103
Cancer therapy has entered a new era; biologically targeted therapies are now commonly used as part of the standard therapy for CRC135
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree