and Michael Camilleri1
(1)
Division of Gastroenterology and Hepatology, Mayo Clinic, Charlton 8, 200 First Street SW, Rochester, MN 55905, USA
Case Study
A 47-year-old woman was referred for further evaluation and management of chronic constipation. Her history was notable for symptoms of constipation-predominant irritable bowel since childhood. When first evaluated, she had a bowel movement once weekly, induced by oral laxatives and/or an enema, with no spontaneous bowel movements. Scintigraphy disclosed normal gastric emptying and small bowel transit but delayed colonic transit. Anorectal manometry and balloon expulsion tests were normal. At that time, her laxative regimen was adjusted.
Five years later, she returned with severe constipation. After no bowel movement for several days, she consumes a large amount of milk of magnesia and bisacodyl. These agents induce severe abdominal cramps and diarrhea followed by temporary relief of constipation. While enemas do not result in passage of stool, she is able to evacuate the enema fluid fairly easily. She denies straining excessively and does not need to use unusual positions on the toilet, has no sense of incomplete evacuation, and does not digitate the rectum or vagina to help evacuate the stool. She also describes right and left lower quadrant abdominal pain that are constant and not always relieved after defecation. Her appetite is fair and her weight has increased 10 lb. in the last 2 years. A colonic motility study with a barostat-manometry assembly was consistent with colonic inertia (see Figs. 17.1 and 17.2). She subsequently underwent laparoscopic colectomy with ileorectostomy. After surgery, she was doing well without laxatives, passing four semi-formed bowel movements daily and having minimal abdominal discomfort.
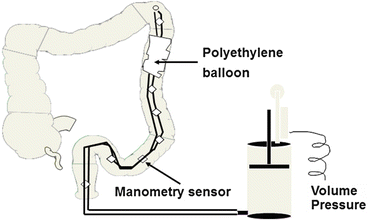
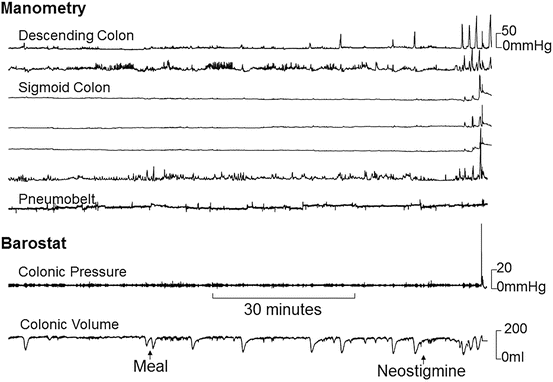

Fig. 17.1
Barostat-manometry assembly positioned in the descending colon with polyethylene balloon in apposition with colonic mucosa

Fig. 17.2
Representative colonic barostat-manometry tracing of colonic inertia. The manometry sensors disclosed increased phasic pressure activity after neostigmine, but not after a meal. There was no change in the baseline volume of a colonic balloon inflated to a fixed pressure after a meal or neostigmine. Taken together, these features indicate colonic inertia
Introduction
Constipation is defined by bowel symptoms, and slow colonic transit is identified by assessing colonic transit time. The Oxford Dictionary defines inertia as “a tendency to do nothing or to remain unchanged.” Colonic inertia refers to markedly reduced or absent colonic contractile responses to physiological (i.e., a meal) and pharmacological stimuli (e.g., bisacodyl or neostigmine) in patients with slow transit constipation. To emphasize, colonic inertia should only be identified by evaluating the colonic contractile response to a meal and a pharmacological stimulus. While slow colonic transit and colonic inertia are often used interchangeably, these terms are not synonymous. Currently, inertia should only be identified by evaluating the colonic contractile response to a meal or neostigmine with intraluminal sensors.
Epidemiology
The incidence, prevalence, and natural history of colonic inertia are unknown. A review of the literature observed that the median prevalence of constipation was 16 % (range 0.7–79 %) in adults overall and 33.5 % in adults aged 60–101 years. There are no reliable population-based estimates of the prevalence of colonic inertia. In a tertiary referral series of 1,411 patients with constipation managed by one gastroenterologist, the proportions of constipated patients who had normal transit constipation (NTC) were ~70 %, slow transit constipation (STC) ~5 %, and pelvic floor dysfunction or defecatory disorders (DD) ~25 %. Even in tertiary centers, only a minority of constipated patients undergoes intraluminal colonic testing; hence, the proportion of constipated patients who truly have colonic inertia is unknown. However, clinical observations and indirect estimates suggest that only a small fraction of patients with chronic constipation have colonic inertia. For example, among 1,009 constipated patients who underwent assessment of colonic transit and anorectal functions, only 52 (5 %) had an abdominal colectomy and ileorectal anastomosis (IRA) for medically refractory slow transit constipation. While colonic motor functions were not evaluated by intraluminal techniques in this study, these 52 patients presumably had severe colonic motor dysfunction refractory to medical management and, possibly, colonic inertia. Our clinical experience suggests that, similar to chronic constipation, the prevalence of colonic inertia is higher in women than in men.
Pathophysiology
Current concepts suggest that the colonic motor dysfunction in colonic inertia may be explained by a marked reduction in colonic intrinsic nerves and interstitial cells of Cajal (ICC), as documented by histopathology. While this is a plausible hypothesis, it must be noted that these histopathological abnormalities were documented in patients with severe chronic constipation or megacolon who did not have intraluminal assessment of colonic motor functions (i.e., with manometry and/or barostat); hence, whether they had colonic inertia is unknown. Moreover, the precise contribution of ICC loss to colonic sensorimotor dysfunction is unclear since other enteric neurons are also depleted or even increased in these conditions. The extent of ICC loss necessary to cause colonic motor dysfunction also remains unknown.
ICC loss can impair colonic motility since these cells regulate gut motility via several mechanisms: they generate electrical slow waves which then propagate through smooth muscle cells via gap junctions; they influence the smooth muscle membrane potential and membrane potential gradient; they convey electrical effects of motor neuron input to smooth muscle; and they mediate some of the mechanosensitivity of smooth muscle. ICC-generated slow waves are triggered by pacemaker currents which evolve into a rapid upstroke when they exceed a depolarization threshold. Slow waves from ICCs spread into smooth muscle via gap junctions and activate a variety of smooth muscle ion channels, including L-type calcium channels, contributing powerfully to the contractile response.
Diagnosis and Evaluation
Intraluminal assessment of colonic motility should be considered in patients with medically refractory slow transit constipation who do not have a defecatory disorder (see Chap. 15). These assessments may reveal colonic motor dysfunction in some patients with slow transit constipation. Manometric disturbances include fewer high amplitude propagated contractions (HAPCs), reduced phasic contractile responses to a meal and/or to pharmacological stimuli (e.g., bisacodyl or neostigmine). A barostat-based evaluation of colonic motor function may reveal reduced fasting tone and/or reduced tonic contractile responses to a meal and/or to pharmacological stimuli (e.g., bisacodyl or neostigmine). In patients with megacolon, compliance of the colon is increased; however, this is not usually observed in colonic inertia in the absence of megacolon. In general, colonic inertia is defined by reduced contractile responses to a meal and a pharmacological stimulus. However, the reduction required to define inertia has not been characterized. Healthy subjects have 1–15 HAPCs daily. Therefore, only patients who have no HAPCs over a 24-h period are truly abnormal.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







