Mesalamine has been the first-line of therapy in patients with inflammatory bowel disease (IBD) since the 1960s. This article serves as a review of the different 5-aminosalicylic acid compounds, release formulations, use and dosing in the treatment of IBD, in particular ulcerative colitis.
The 5-aminosalicylic acid (5-ASA, mesalamine) class of drugs has been recommended as the first line of therapy in patients with mild to moderate ulcerative colitis (UC). Mesalamine’s mechanism of action is not fully understood, but it has been shown to be successful in the induction and maintenance of clinical remission in patients with UC. Since the discovery of sulfasalazine, there have been several newer preparations of 5-ASA with different modes of delivery on the market, all aimed at minimal systemic absorption and maximal topical application.
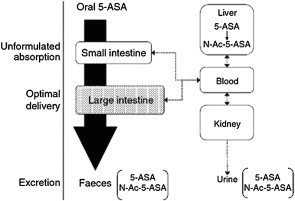
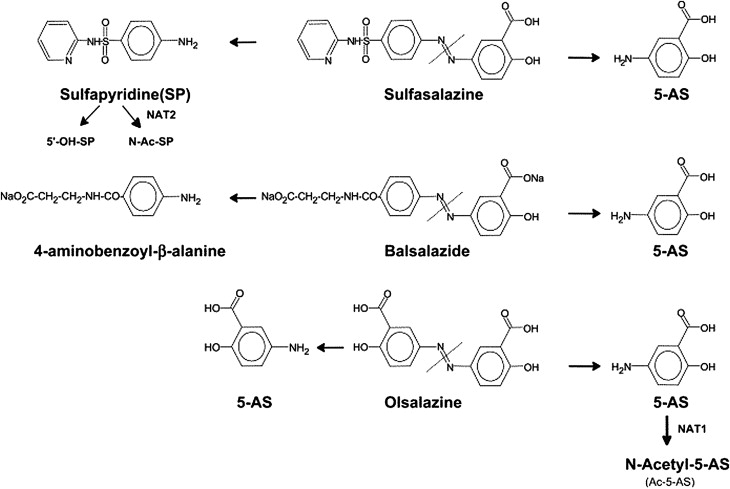
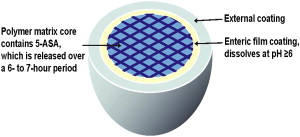
Rectal administration of gels, foams, and enemas containing 5-ASA is the most effective way to achieve maximal topical efficacy. Topical mesalamine has been demonstrated to be effective in treating patients with active proctosigmoiditis and left-sided colitis. However, patient tolerability of rectal administration is poor because of difficulty in administration, problems with retention and leakage, and patient discomfort. Thus, despite evidence suggesting a superiority of topical mesalamine to oral aminosalicylates alone in achieving clinical improvement in patients with mild to moderate distal colitis, the therapeutic plan is often guided largely by patient preferences with the oral route of administration preferred over rectal therapy. Oral 5-ASA in its unformulated form is rapidly absorbed in the small intestine, leaving a minimal concentration of drug to treat the colon ( Fig. 1 ). Various formulations have thus been manufactured to ensure maximal delivery of 5-ASA to the site of inflammation. Sulfasalazine, olsalazine, and balsalazide are azo-bonded prodrugs, in which 5-ASA (the active moiety) is linked by an azo bond to the carrier moiety ( Fig. 2 ). The bond is cleaved by colonic bacterial azoreductase, resulting in release of the 2 constituent moieties. Other oral formulations such as Asacol, which is coated with a gastroresistant and a pH-sensitive acrylic-based resin (Eudragit-S), delay release of the drug until it reaches the terminal ileum, where the pH is consistently 7 or higher. Similarly, Apriso also consists of a delayed-release mechanism and offers once-daily dosing with a capsule containing granules composed of mesalamine in a polymer matrix with an enteric (outer) coating, Eudragit-L. The outer coating dissolves in the terminal ileum (pH ≥6), while the polymer matrix core facilitates slow, sustained release throughout the colon ( Fig. 3 ). Pentasa, formulated as ethylcellulose-coated controlled-release capsules, is released throughout the gastrointestinal tract. Lialda contains a “Multi Matrix System Technology” (MMX) with lipophilic and hydrophilic matrices to provide delayed release of mesalamine. Lialda contains Eudragit-S, which enables mesalamine release at pH 7 or higher in the terminal ileum, and its release continues throughout the colon. Despite the variety of delivery mechanisms employed, the results of clinical trials have shown the efficacy of historically available oral formulations to be broadly similar. This review focuses on a more detailed discussion of the various 5-ASA preparations and release formulations, to guide physicians in the clinical decisions regarding choice of drug.
Mechanism of action
Mesalamine’s effectiveness is due to its anti-inflammatory properties. The drug blocks interleukin-1 and tumor necrosis factor-α (TNF-α). The drug inhibits binding of TNF-α to its receptor, preventing downstream signaling. This process leads to downstream inhibition of nuclear factor-κB, which has been detected in inflamed mucosa in inflammatory bowel disease (IBD). It has been hypothesized that 5-ASA also activates peroxisome proliferator-activated receptors in colonic epithelial cells, which are involved in the control of inflammation, cell proliferation, apoptosis, and metabolic function.
The drug also inhibits the cyclooxygenase pathway, leading to inhibition of prostaglandin E 2 in inflamed intestine. Also, its blocking of the lipooxygenase pathway inhibits the production of leukotrienes. 5-ASA may also act as a scavenger of free radicals, thus having antioxidant properties.
Preparations
As 5-ASA exerts its effect directly on the colonic mucosa, it is most effective if it is administered via the rectal route using enemas, gels, or foams. This method allows the drug to reach the rectum, sigmoid, and left side of the colon. However, due to discomfort, retention, and leakage associated with the 5-ASA enema preparation, patient compliance has been a major concern, which has been particularly appreciated during treatment of patients with active UC.
Oral administration of 5-ASA in its simplest form is not effective because the majority of the drug is absorbed by the small bowel, resulting in insufficient concentration of drug by the time it reaches the large intestine. In addition, acetylation in the gut epithelium and liver inactivates the drug. Sulfasalazine was the first aminosalicylate to be used for the treatment of patients with IBD; however, it was associated with many adverse effects and frequent allergic reactions. The majority were attributable to its carrier moiety, sulfapyridine. To address this problem, new oral formulations have been developed that allow enough drug to reach the distal bowel in therapeutic concentrations. Mesalamine, olsalazine, and balsalazide are the other 3 available oral preparations of 5-ASA. A summary of various 5-ASA formulations is presented in Table 1 .
| Name Formulation | Release Site | Dosage Per Tablet |
|---|---|---|
| Sulfasalazine/Azulfidine | 5-ASA linked to sulfapyridine by an azo bond | 500 mg |
| Asacol/delayed release mesalamine | Enclosed in enteric “film” (Eudragit-S) releasing at pH ≥7 in the terminal ileum and colon | 400 mg (Asacol) |
| 800 mg (Asacol HD) | ||
| Salofalk, Claversal | Enclosed Eudragit-L releasing at pH ≥6 in the jejunum, ileum, and colon | 250 mg (Salofalk) 500 mg (Salofalk and Claversal) |
| Pentasa | Microspheres within a moisture-sensitive, ethylcellulose, semipermeable membrane releasing mesalamine in the duodenum, jejunum, ileum, and colon | 250 mg and 500 mg capsules |
| Apriso | The outer coating (Eudragit-L) dissolves in the jejunum, ileum, and colon (pH ≥6), while a polymer matrix core facilitates slow, sustained release throughout the colon | 375 mg |
| Olsalazine/Dipentum | Two molecules of 5-ASA linked by an azo bond between their amino groups cleaved by azoreductase | 250 mg |
| Balsalazide disodium/Colazal | 5-ASA linked by an azo bond to 4-amino-benzoyl-β-alanine cleaved by azoreductase | 750 mg |
| Lialda, Mezavant | Has lipophilic and hydrophilic matrices to provide delayed release of mesalamine. Has Eudragit-S, which enables mesalamine release at pH ≥7 in the terminal ileum and continues throughout the colon | 1200 mg |
| Salofalk Granustix, Pentasa sachets | Micropellet formulations | 500 mg sachets |
The mucosal concentration of 5-ASA is inversely correlated with severity of inflammation in patients with UC. Patients with none or mild endoscopic and microscopic colonic inflammation had significantly higher mucosal concentrations of 5-ASA when compared with those having moderate and severe macroscopic ( P = .03) and microscopic ( P <.01) activity of UC. On the other hand, levels of soluble interleukin-2 receptor, which is a proinflammatory cytokine, were significantly lower in patients with high mucosal concentrations of 5-ASA when compared with those with low mucosal concentrations. A study of 18 patients with UC and high risk of relapse has demonstrated that increased 5-ASA doses lead to an improved clinical course of UC. It has been suggested that mucosal concentrations of 5-ASA depend not only on the dose of the drug but also on other factors. A phase 2 study of MMX mesalamine observed higher 5-ASA mucosal concentrations in the sigmoid colon and rectum in patients with UC after treatment at a once-daily dose of 4.8 g when compared with one daily dose of 1.2 or 2.4 g. On the other hand, a biopsy study has demonstrated that patients with UC treated with balsalazide at the daily dose of 6.75 g (equivalent to pH dependent mesalamine at the daily dose of 2.4 g) had a 96% higher mean mucosal concentrations of 5-ASA in the distal colon than in those receiving pH-dependent mesalamine at the daily dose of 3.74 g. Therefore, mucosal concentrations of 5-ASA may depend on its formulation and be independent of dose-plasma concentration relationships. Studies have demonstrated interindividual variability in 5-ASA mucosal concentrations, which may be caused by local pH conditions, transit rates, and activities of enzymes and transporters involved in metabolism of 5-ASA within colonic mucosa. It has been suggested that the mucosal concentration of 5-ASA might be inversely related to the risk of relapse in patients receiving maintenance treatment.
Sulfasalazine
Sulfasalazine/Azulfidine (Pharmacia & Upjohn Co, New York) consists of 5-ASA linked to sulfapyridine by an azo bond (see Fig. 2 ). The bond is cleaved by colonic bacterial azoreductase. In 1977, Azad Khan and colleagues published data of a nonplacebo controlled trial to discern if sulfasalazine’s efficacy is a result of the parent molecule or its metabolites, sulfapyridine and 5-ASA. The study consisted of a blind nonplacebo controlled trial in patients with UC given retention enemas of sulfasalazine, sulfapyridine, or 5-ASA daily for 2 weeks. The primary end point was histologic improvement, which was observed in 30% of patients receiving sulfasalazine or 5-ASA, but only 5% of those receiving sulfapyridine. Thus, the study concluded that the active moiety was the 5-ASA metabolite of sulfasalazine. In addition, the sulfapyridine moiety is absorbed systemically, leading to adverse effects, whereas the 5-ASA moiety is less well absorbed from the distal small intestine and thus reaches high concentrations (1–100 mmol/L) in the lumen of the colon and rectum.
Mesalamine
Mesalamine (termed mesalazine in Europe) is otherwise known as 5-ASA.
Formulations
There are various formulations of mesalamine enclosed within an enteric coat or “film” such as Eudragit-S (used in Asacol and Lialda) or Eudragit-L (used in Apriso) (Röhm GmbH & Co KG, Pharma Polymers, Darmstadt, Germany). The enteric coating (Eudragit-L) is broken down in the jejunum, terminal ileum, or cecum when a change in pH (at ≥6) causes it to disintegrate and release the active drug. Similarly, the enteric coating (Eudragit-S) is broken down in the terminal ileum, or cecum when a change in pH (at ≥7) causes it to disintegrate and release the active drug. Asacol (Procter & Gamble Pharmaceuticals, Cincinnati, OH, USA), Salofalk (Axcan Pharma, Mont St Hilaire, Quebec, Canada and Falk Pharma, Freiburg, Germany), Claversal (Merckle GMbH, Ulm, Germany), Mesasal (GlaxoSmithKline, Mississauga, ON, Canada), Ipocol (Sandoz Pharmaceuticals, Bordon, Hampshire, UK), and Mesren (Ivax Pharmaceuticals Limited, Runcorn, Cheshire, UK), use the enteric coated approach.
Pentasa (Shire Pharmaceuticals Inc, Wayne, PA, USA, licensed from Ferring A/S, Copenhagen, Denmark) is another mesalamine-containing drug, but instead of having an enteric coat it is composed of microspheres containing 5-ASA enclosed within a moisture-sensitive, ethylcellulose, semipermeable membrane, allowing for pH-independent release of the drug. Drug release begins in the duodenum (unlike the other drugs mentioned, which release further down the bowel).
Apriso (Salix Pharmaceuticals Inc, Morrisville, NC, USA) is composed of gelatin capsules that dissolve, releasing granules of the drug into the stomach. The delayed-release coating (Eudragit-L) on the granules dissolves at a pH of 6 or higher. The granules contain an extended-release polymer matrix core that swells and gradually distributes mesalamine throughout the colon. Apriso is the newest of the 5-ASAs to be approved by the Food and Drug Administration (FDA), with the main benefit of it being administered once a day. This medication is currently FDA approved for maintenance of remission for patients with ulcerative colitis. Two randomized, double-blind, placebo-controlled, multicenter trials were conducted on patients in remission from mild to moderate UC where Apriso was given at 1.5 g/d and compared with placebo. The primary end point was remaining relapse free at 6 months (defined using Sutherland Disease Activity Index [DAI] of rectal bleeding subscale score of ≥1 and mucosal appearance score ≥2). Of the 562 patients enrolled, 70% ( P = .001) were relapse-free at month 6 compared with 56% in the placebo arm. The recommended dose is 4 0.375-g capsules once daily (a total of 1.5 g daily). Adverse effects include renal impairment, headache, diarrhea, upper abdominal pain, nausea, nasopharyngitis, influenza, influenza-like illness, and sinusitis. Hepatic failure was very rarely noted to occur in patients with preexisting liver disease.
Olsalazine/Dipentum (Alaven Pharmaceuticals, Marietta, GA) consists of 2 molecules of 5-ASA linked by an azo bond between their amino groups. The azo bond is split by bacterial azoreductase in the distal small bowel and colon, releasing the active moiety (see Fig. 2 ).
Benzalazine, balsalazide/Colazide (Astra Zeneca), and balsalazide disodium/Colazal (Salix Pharmaceuticals) is comprised of 5-ASA linked by an azo bond to 4-amino-benzoy-β-alanine which, similar to Olsalazine, is cleaved by bacterial azoreductase in the distal bowel, thus releasing the active 5-ASA. The 4-amino-benzoy-β-alanine is inert and poorly absorbed, causing few side effects (see Fig. 2 ).
The MMX technology (previously known as SPD476, now marketed as Lialda in the United States and Mezavant in Canada; Shire Pharmaceuticals Inc, Wayne, PA) is a high-strength formulation of 5-ASA (1.2 g per tablet). This system uses lipophilic and hydrophilic matrices, all enclosed within a gastroresistant, pH-dependent coating (Eudragit-S), allowing drug release at pH 7 or higher in the terminal ileum. After the coating disintegrates, an interaction between the hydrophilic matrix and intestinal fluids occurs leading to swelling of the drug and formation of a viscous gel mass allowing for slow diffusion and release of active drug down the colon. The hydrophilic matrix also adheres to the colonic mucosa. Several randomized placebo-controlled studies have investigated the efficacy and safety of the MMX formulation in comparison with placebo. In 2007, Kamm and colleagues published a double-blind, multicenter study comparing MMX mesalamine to placebo for treatment of active UC (with an Asacol internal reference arm). Patients with active mild to moderate UC (n = 343) who received 2.4 g/d or 4.8 g/d of MMX once daily, Asacol 2.4 g/d divided into 3 doses, or placebo for 8 weeks. The study reported 40.5% ( P = .01) of the 2.4 g/d MMX and 41.2% ( P = .007) of the 4.8 g/d MMX achieved clinical and endoscopic remission at week 8, compared with 22.1% in the placebo group. In addition, Sandborn and colleagues pooled data from 2 phase 3, randomized placebo-controlled trials assessing the efficacy of MMX for inducing remission of mild to moderately active UC performed by Kamm and colleagues and Lichtenstein and colleagues. A total of 517 patients were randomized to receive 8 weeks of therapy with either MMX 2.4 g/d (every day or divided twice daily (n = 172), MMX 4.8 g/d (every day) (n = 174) or placebo (n = 171). The proportion of patients in remission (defined as UC DAI ≤1) at week 8 was 2-fold higher in the MMX treated arm regardless of dose (37.2% for lower dose and 35.1% for higher dose) when compared with placebo (17.5%, P <.001). Likewise, patients treated with MMX had 2-fold higher rates of complete mucosal healing than placebo recipients at week 8 (32% vs 16%, P value-not reported). The percentages in remission were significantly greater ( P <.05) in the MMX group compared with placebo when stratified for disease extent, disease severity, and gender as well as among patients not previously receiving low-dose ASA. MMX at 4.8 g/d was also more successful than placebo in patients transferring from prior low-dose oral 5-ASA, but not on 2.5 g/d MMX. The study concluded that MMX mesalamine was efficacious for active UC regardless of disease extent, severity, gender, and previous low-dose 5-ASA therapy. Adverse effects of MMX included abnormal liver function tests, angina pectoris, pulmonary edema, cerebral infarction, abdominal pain, diarrhea, flatulence, nausea, nasopharyngitis, and headache. The safety profile of MMX is similar to that of other oral nonsulfa-5-ASA therapies. The clinical studies demonstrate that MMX mesalamine (at either 2.4 g/d or 4.8 g/d dose) is effective for inducing clinical and endoscopic remission in patients with active mild to moderate UC. For patients who do not respond to initial 8 weeks of therapy benefit from an additional 8 weeks of therapy. Extended therapy beyond 8 weeks may prevent escalation to steroids or biologics. MMX mesalamine is successful in maintaining remission for at least 12 months. Given MMX mesalamine’s simplified dosing regimen and ability to induce and maintain clinical and endoscopic remission in patients with mild to moderate UC, this formulation is commonly recommended to patients with active UC. Lower pill burden aids compliance and, hopefully, better clinical outcomes. Prospective, community-based studies will evaluate adherence to MMX mesalamine. Trials comparing the various 5-ASA formulations are needed to further evaluate the efficacy of these different medications.
Salofalk Granustix sachets (Falk Pharma) and Pentasa sachets (Ferring Pharmaceuticals) are micropellet formulations of 5-ASA that allow for less frequent dosing and an easy-to-swallow drug. The Salofalk granustix sachets release 5-ASA in the ileocecal region, which is similar to the Salofalk tablets; however, there is a prolonged release of 5-ASA from the sachet compared with the tablet. The Pentasa sachets contain mesalamine and also have a prolonged release; the drug is released in the terminal ileum and ascending colon.
Dosing of mesalamine
Dose response studies for mild to moderate active UC reported additional benefit of 2.4 g/d compared with 1.6 g/d, and another study showed added benefit of 4.8 g/d dosing compared with 1.6 g/d. Two meta-analyses recommended dosing 3 g/d or greater for mild to moderate active UC. The regimen commonly used by clinicians is to administer a 2.4 g/d dose for mild to moderate active UC, with an increase to a maximum of 4.8 g/d if patients are not responsive to the lower dose. The Assessing the Safety & Clinical Efficacy of New Dose of 5-ASA (ASCEND) trials involved 2 randomized, double-blind placebo-controlled trials comparing 4.8 g and 2.4 g/d dosing of mesalamine. The ASCEND I trial involved 301 patients with mild to moderately active UC with a primary end point of overall improvement (complete remission or clinical response to therapy) at week 6 of treatment in the format of a noninferiority trial. The results revealed significant improvement on the higher dose compared with the lower dose for those with moderately active UC (57% in 2.4 g/d, 72.4% in 4.8 g/d; P = .0384). The ASCEND II trial involved moderately active UC only (286 patients), and patients were randomized to the 2.4 g/d dose or 4.8 g/d daily dose of mesalamine; the primary end point was the same as that of the ASCEND I trial. Results yielded a significant treatment success in those on the 4.8 g/d dose (72%, 89/124 patients) compared with those on the 2.4 g/d dose (59%, 77/130 patients, P = .036). Adverse events were similar in both groups. The studies suggested that the higher dose is more effective at providing acute response and remission in patients with moderately active UC, while the lower dose is sufficient in patients with mildly active UC.
The notion that a higher dose (4.8 g of mesalamine) is superior to 2.4 g of mesalamine is not true in all populations studied. The primary end point of the ASCEND II and III studies was overall improvement at 6 weeks, which was determined by the Physician’s Global Assessment (PGA), which encompassed the clinical assessments of rectal bleeding, stool frequency, and sigmoidoscopy findings.
In ASCEND III, only patients with moderately active UC were studied. Overall, 70% of patients (n = 273/389) achieved overall improvement with Asacol HD at 4.8 g/d compared with 66% of patients (n = 251/383) who took Asacol 400 mg tablets at 2.4 g/d. (In ASCEND II, 72% of patients [n = 89/124] achieved overall improvement with Asacol HD at 4.8 g/d at 6 weeks, as compared with 59% of patients [n = 77/130] who took Asacol 400 mg tablets at 2.4 g/d [ P = .036]). Thus, when compared with each other, 2.4 g of mesalamine in the formulation of delayed-release mesalamine has similar efficacy as compared with 4.8 g of mesalamine for active treatment of ulcerative colitis. In a post hoc analysis there is, however, a suggestion that certain subsets of patients may benefit from high dose (4.8 g of mesalamine). In particular, in the ASCEND III trial there is a therapeutic advantage for the 4.8 g/d dose compared with the 2.4 g daily dose observed among patients previously treated with corticosteroids, oral mesalamines, rectal therapies, or multiple UC medications.
The convention that dosing 5-ASA compounds multiple times a day was challenged initially by studies of Kamm colleagues and Lichtenstein colleagues using MMX mesalamine in assessing efficacy of induction of response and remission. Similarly, a once daily formulation was found to be effective for maintenance of remission by using Apriso for maintenance of remission in 2 6-month maintenance studies.
The same findings were reproduced in a study evaluating delayed release mesalamine by Sandborn and colleagues. This multicenter, randomized, investigator-blinded, 12-month, active-control trial involved 1023 patients and compared once daily (QD) dosing to twice daily (BID) dosing of delayed-release mesalamine (Asacol) 1.6 to 2.4 g/d in patients with UC and who were currently in clinical remission (for at least 3 months). The primary end point was maintenance of clinical remission at 6 months. At month 6, 90.5% (428/483) of patients on the QD dose and 91.8% (435/474) on the BID dose maintained remission (95% confidence interval [CI] for BID-QD, −2.3, 4.9, respectively). The study was designed to be a noninferiority design trial. At month 12, 85.4% (379/444) or patients on the QD dose and 85.4% (380/445) of the BID dose maintained clinical remission (95% CI for BID-QD, −4.6, 4.7 respectively). Withdrawals due to adverse events were similar in both groups. The study concluded that once-daily dosing of Asacol at doses of 1.6 to 2.4 g/d was effective for the maintenance of clinical remission in patients with UC.
Foam versus liquid enema
Rectal administration of 5-ASA has been advocated as the treatment of choice in proctitis, proctosigmoiditis, and left-sided UC because local concentrations and active drug at the affected site are sufficiently high for therapeutic activity, and in addition, systemic absorption is considerably lower. Other advantages include a generally quicker response time and less frequent dosing schedule. Different meta-analyses of controlled trials have also indicated a superiority of topical mesalamine over oral 5-ASA alone in achieving clinical improvement in patients with mild to moderate distal colitis. However, in practice, rectal application often serves as an alternative or add-on therapy to oral 5-ASA.
Mesalamine rectal suppositories have been shown effective in the treatment of proctitis and maintenance of remission; however, their use is limited because they do not provide sufficient spread of the active ingredient beyond the distal colon. The spread of mesalamine liquid enema formulation is generally good, and has been shown to be effective in inducing and maintaining remission in distal colitis. However, patient acceptance may be compromised by difficulties with self-administration, retention, discomfort, or the necessity for prolonged bed rest. Rectal mesalamine foam was developed to optimize drug delivery and improve patient acceptance. The rectal foam has a higher viscosity than the liquid enema, which favors retention, enhances mucosal adhesion, and provides wider mucosal spread.
An internal pharmaceutical company report assessed the extent of dispersion of the rectal foam in a gamma scintigraphic study in healthy volunteers. It was found that immediately after dosing, the foam had spread through the rectum and sigmoid colon, reaching the upper descending colon after 12 hours.
Pokrotnieks and colleagues in a multicenter, randomized, double-blind parallel-group study showed that mesalamine foam was well tolerated and more effective than placebo in patients with distal UC. In this study, 111 patients with mild to moderately active proctitis, proctosigmoiditis, or left-sided UC received mesalamine foam or placebo enema for 6 weeks. Clinical remission was more frequent in the mesalamine than the placebo group (65% vs 40%; P = .0082). The frequency of endoscopic remission was also higher in the mesalamine group (57%) than in the placebo group (37%). In comparing the efficacy of mesalamine foam with mesalamine liquid enema, Cortot and colleagues conducted a multicenter investigator-blinded clinical trial and randomized 375 patients with mild to moderate UC to rectal foam or liquid enema for 4 weeks. Eligible patients were 18 years and older with newly diagnosed or relapsing active mild to moderate left-sided UC, with a disease extension of at least 5 cm from the anal margin and not above the splenic flexure. Remission rates at week 4 in mesalamine foam versus mesalamine liquid enema were 68.3% versus 73.6% in the per protocol (PP) population (lower limit of 97.5% CI −15.1%) and 66.7% versus 70.5% in the intention-to-treat (ITT) population (97.5% CI −13.4%). Remission rates at week 2 were 48.1% versus 50.6% in ITT (97.5% CI −12.8%) and 49.1% versus 52.1% in PP (97.5% CI −13.8%) in foam versus liquid enema, respectively. Both treatments were well tolerated with minimal adverse events. Although the noninferiority of mesalamine foam could not be strictly demonstrated at week 4 in the PP analysis, it was achieved in the ITT population and at week 2 in both populations. Mesalamine foam represents a clinically efficient and well tolerated therapeutic alternative to mesalamine liquid enema in patients with mild to moderately active proctitis and proctosigmoiditis. It may be especially appropriate in cases of poor tolerance of enemas because of acute rectal inflammation.
Preparations
As 5-ASA exerts its effect directly on the colonic mucosa, it is most effective if it is administered via the rectal route using enemas, gels, or foams. This method allows the drug to reach the rectum, sigmoid, and left side of the colon. However, due to discomfort, retention, and leakage associated with the 5-ASA enema preparation, patient compliance has been a major concern, which has been particularly appreciated during treatment of patients with active UC.
Oral administration of 5-ASA in its simplest form is not effective because the majority of the drug is absorbed by the small bowel, resulting in insufficient concentration of drug by the time it reaches the large intestine. In addition, acetylation in the gut epithelium and liver inactivates the drug. Sulfasalazine was the first aminosalicylate to be used for the treatment of patients with IBD; however, it was associated with many adverse effects and frequent allergic reactions. The majority were attributable to its carrier moiety, sulfapyridine. To address this problem, new oral formulations have been developed that allow enough drug to reach the distal bowel in therapeutic concentrations. Mesalamine, olsalazine, and balsalazide are the other 3 available oral preparations of 5-ASA. A summary of various 5-ASA formulations is presented in Table 1 .
| Name Formulation | Release Site | Dosage Per Tablet |
|---|---|---|
| Sulfasalazine/Azulfidine | 5-ASA linked to sulfapyridine by an azo bond | 500 mg |
| Asacol/delayed release mesalamine | Enclosed in enteric “film” (Eudragit-S) releasing at pH ≥7 in the terminal ileum and colon | 400 mg (Asacol) |
| 800 mg (Asacol HD) | ||
| Salofalk, Claversal | Enclosed Eudragit-L releasing at pH ≥6 in the jejunum, ileum, and colon | 250 mg (Salofalk) 500 mg (Salofalk and Claversal) |
| Pentasa | Microspheres within a moisture-sensitive, ethylcellulose, semipermeable membrane releasing mesalamine in the duodenum, jejunum, ileum, and colon | 250 mg and 500 mg capsules |
| Apriso | The outer coating (Eudragit-L) dissolves in the jejunum, ileum, and colon (pH ≥6), while a polymer matrix core facilitates slow, sustained release throughout the colon | 375 mg |
| Olsalazine/Dipentum | Two molecules of 5-ASA linked by an azo bond between their amino groups cleaved by azoreductase | 250 mg |
| Balsalazide disodium/Colazal | 5-ASA linked by an azo bond to 4-amino-benzoyl-β-alanine cleaved by azoreductase | 750 mg |
| Lialda, Mezavant | Has lipophilic and hydrophilic matrices to provide delayed release of mesalamine. Has Eudragit-S, which enables mesalamine release at pH ≥7 in the terminal ileum and continues throughout the colon | 1200 mg |
| Salofalk Granustix, Pentasa sachets | Micropellet formulations | 500 mg sachets |
The mucosal concentration of 5-ASA is inversely correlated with severity of inflammation in patients with UC. Patients with none or mild endoscopic and microscopic colonic inflammation had significantly higher mucosal concentrations of 5-ASA when compared with those having moderate and severe macroscopic ( P = .03) and microscopic ( P <.01) activity of UC. On the other hand, levels of soluble interleukin-2 receptor, which is a proinflammatory cytokine, were significantly lower in patients with high mucosal concentrations of 5-ASA when compared with those with low mucosal concentrations. A study of 18 patients with UC and high risk of relapse has demonstrated that increased 5-ASA doses lead to an improved clinical course of UC. It has been suggested that mucosal concentrations of 5-ASA depend not only on the dose of the drug but also on other factors. A phase 2 study of MMX mesalamine observed higher 5-ASA mucosal concentrations in the sigmoid colon and rectum in patients with UC after treatment at a once-daily dose of 4.8 g when compared with one daily dose of 1.2 or 2.4 g. On the other hand, a biopsy study has demonstrated that patients with UC treated with balsalazide at the daily dose of 6.75 g (equivalent to pH dependent mesalamine at the daily dose of 2.4 g) had a 96% higher mean mucosal concentrations of 5-ASA in the distal colon than in those receiving pH-dependent mesalamine at the daily dose of 3.74 g. Therefore, mucosal concentrations of 5-ASA may depend on its formulation and be independent of dose-plasma concentration relationships. Studies have demonstrated interindividual variability in 5-ASA mucosal concentrations, which may be caused by local pH conditions, transit rates, and activities of enzymes and transporters involved in metabolism of 5-ASA within colonic mucosa. It has been suggested that the mucosal concentration of 5-ASA might be inversely related to the risk of relapse in patients receiving maintenance treatment.
Sulfasalazine
Sulfasalazine/Azulfidine (Pharmacia & Upjohn Co, New York) consists of 5-ASA linked to sulfapyridine by an azo bond (see Fig. 2 ). The bond is cleaved by colonic bacterial azoreductase. In 1977, Azad Khan and colleagues published data of a nonplacebo controlled trial to discern if sulfasalazine’s efficacy is a result of the parent molecule or its metabolites, sulfapyridine and 5-ASA. The study consisted of a blind nonplacebo controlled trial in patients with UC given retention enemas of sulfasalazine, sulfapyridine, or 5-ASA daily for 2 weeks. The primary end point was histologic improvement, which was observed in 30% of patients receiving sulfasalazine or 5-ASA, but only 5% of those receiving sulfapyridine. Thus, the study concluded that the active moiety was the 5-ASA metabolite of sulfasalazine. In addition, the sulfapyridine moiety is absorbed systemically, leading to adverse effects, whereas the 5-ASA moiety is less well absorbed from the distal small intestine and thus reaches high concentrations (1–100 mmol/L) in the lumen of the colon and rectum.
Mesalamine
Mesalamine (termed mesalazine in Europe) is otherwise known as 5-ASA.
Formulations
There are various formulations of mesalamine enclosed within an enteric coat or “film” such as Eudragit-S (used in Asacol and Lialda) or Eudragit-L (used in Apriso) (Röhm GmbH & Co KG, Pharma Polymers, Darmstadt, Germany). The enteric coating (Eudragit-L) is broken down in the jejunum, terminal ileum, or cecum when a change in pH (at ≥6) causes it to disintegrate and release the active drug. Similarly, the enteric coating (Eudragit-S) is broken down in the terminal ileum, or cecum when a change in pH (at ≥7) causes it to disintegrate and release the active drug. Asacol (Procter & Gamble Pharmaceuticals, Cincinnati, OH, USA), Salofalk (Axcan Pharma, Mont St Hilaire, Quebec, Canada and Falk Pharma, Freiburg, Germany), Claversal (Merckle GMbH, Ulm, Germany), Mesasal (GlaxoSmithKline, Mississauga, ON, Canada), Ipocol (Sandoz Pharmaceuticals, Bordon, Hampshire, UK), and Mesren (Ivax Pharmaceuticals Limited, Runcorn, Cheshire, UK), use the enteric coated approach.
Pentasa (Shire Pharmaceuticals Inc, Wayne, PA, USA, licensed from Ferring A/S, Copenhagen, Denmark) is another mesalamine-containing drug, but instead of having an enteric coat it is composed of microspheres containing 5-ASA enclosed within a moisture-sensitive, ethylcellulose, semipermeable membrane, allowing for pH-independent release of the drug. Drug release begins in the duodenum (unlike the other drugs mentioned, which release further down the bowel).
Apriso (Salix Pharmaceuticals Inc, Morrisville, NC, USA) is composed of gelatin capsules that dissolve, releasing granules of the drug into the stomach. The delayed-release coating (Eudragit-L) on the granules dissolves at a pH of 6 or higher. The granules contain an extended-release polymer matrix core that swells and gradually distributes mesalamine throughout the colon. Apriso is the newest of the 5-ASAs to be approved by the Food and Drug Administration (FDA), with the main benefit of it being administered once a day. This medication is currently FDA approved for maintenance of remission for patients with ulcerative colitis. Two randomized, double-blind, placebo-controlled, multicenter trials were conducted on patients in remission from mild to moderate UC where Apriso was given at 1.5 g/d and compared with placebo. The primary end point was remaining relapse free at 6 months (defined using Sutherland Disease Activity Index [DAI] of rectal bleeding subscale score of ≥1 and mucosal appearance score ≥2). Of the 562 patients enrolled, 70% ( P = .001) were relapse-free at month 6 compared with 56% in the placebo arm. The recommended dose is 4 0.375-g capsules once daily (a total of 1.5 g daily). Adverse effects include renal impairment, headache, diarrhea, upper abdominal pain, nausea, nasopharyngitis, influenza, influenza-like illness, and sinusitis. Hepatic failure was very rarely noted to occur in patients with preexisting liver disease.
Olsalazine/Dipentum (Alaven Pharmaceuticals, Marietta, GA) consists of 2 molecules of 5-ASA linked by an azo bond between their amino groups. The azo bond is split by bacterial azoreductase in the distal small bowel and colon, releasing the active moiety (see Fig. 2 ).
Benzalazine, balsalazide/Colazide (Astra Zeneca), and balsalazide disodium/Colazal (Salix Pharmaceuticals) is comprised of 5-ASA linked by an azo bond to 4-amino-benzoy-β-alanine which, similar to Olsalazine, is cleaved by bacterial azoreductase in the distal bowel, thus releasing the active 5-ASA. The 4-amino-benzoy-β-alanine is inert and poorly absorbed, causing few side effects (see Fig. 2 ).
The MMX technology (previously known as SPD476, now marketed as Lialda in the United States and Mezavant in Canada; Shire Pharmaceuticals Inc, Wayne, PA) is a high-strength formulation of 5-ASA (1.2 g per tablet). This system uses lipophilic and hydrophilic matrices, all enclosed within a gastroresistant, pH-dependent coating (Eudragit-S), allowing drug release at pH 7 or higher in the terminal ileum. After the coating disintegrates, an interaction between the hydrophilic matrix and intestinal fluids occurs leading to swelling of the drug and formation of a viscous gel mass allowing for slow diffusion and release of active drug down the colon. The hydrophilic matrix also adheres to the colonic mucosa. Several randomized placebo-controlled studies have investigated the efficacy and safety of the MMX formulation in comparison with placebo. In 2007, Kamm and colleagues published a double-blind, multicenter study comparing MMX mesalamine to placebo for treatment of active UC (with an Asacol internal reference arm). Patients with active mild to moderate UC (n = 343) who received 2.4 g/d or 4.8 g/d of MMX once daily, Asacol 2.4 g/d divided into 3 doses, or placebo for 8 weeks. The study reported 40.5% ( P = .01) of the 2.4 g/d MMX and 41.2% ( P = .007) of the 4.8 g/d MMX achieved clinical and endoscopic remission at week 8, compared with 22.1% in the placebo group. In addition, Sandborn and colleagues pooled data from 2 phase 3, randomized placebo-controlled trials assessing the efficacy of MMX for inducing remission of mild to moderately active UC performed by Kamm and colleagues and Lichtenstein and colleagues. A total of 517 patients were randomized to receive 8 weeks of therapy with either MMX 2.4 g/d (every day or divided twice daily (n = 172), MMX 4.8 g/d (every day) (n = 174) or placebo (n = 171). The proportion of patients in remission (defined as UC DAI ≤1) at week 8 was 2-fold higher in the MMX treated arm regardless of dose (37.2% for lower dose and 35.1% for higher dose) when compared with placebo (17.5%, P <.001). Likewise, patients treated with MMX had 2-fold higher rates of complete mucosal healing than placebo recipients at week 8 (32% vs 16%, P value-not reported). The percentages in remission were significantly greater ( P <.05) in the MMX group compared with placebo when stratified for disease extent, disease severity, and gender as well as among patients not previously receiving low-dose ASA. MMX at 4.8 g/d was also more successful than placebo in patients transferring from prior low-dose oral 5-ASA, but not on 2.5 g/d MMX. The study concluded that MMX mesalamine was efficacious for active UC regardless of disease extent, severity, gender, and previous low-dose 5-ASA therapy. Adverse effects of MMX included abnormal liver function tests, angina pectoris, pulmonary edema, cerebral infarction, abdominal pain, diarrhea, flatulence, nausea, nasopharyngitis, and headache. The safety profile of MMX is similar to that of other oral nonsulfa-5-ASA therapies. The clinical studies demonstrate that MMX mesalamine (at either 2.4 g/d or 4.8 g/d dose) is effective for inducing clinical and endoscopic remission in patients with active mild to moderate UC. For patients who do not respond to initial 8 weeks of therapy benefit from an additional 8 weeks of therapy. Extended therapy beyond 8 weeks may prevent escalation to steroids or biologics. MMX mesalamine is successful in maintaining remission for at least 12 months. Given MMX mesalamine’s simplified dosing regimen and ability to induce and maintain clinical and endoscopic remission in patients with mild to moderate UC, this formulation is commonly recommended to patients with active UC. Lower pill burden aids compliance and, hopefully, better clinical outcomes. Prospective, community-based studies will evaluate adherence to MMX mesalamine. Trials comparing the various 5-ASA formulations are needed to further evaluate the efficacy of these different medications.
Salofalk Granustix sachets (Falk Pharma) and Pentasa sachets (Ferring Pharmaceuticals) are micropellet formulations of 5-ASA that allow for less frequent dosing and an easy-to-swallow drug. The Salofalk granustix sachets release 5-ASA in the ileocecal region, which is similar to the Salofalk tablets; however, there is a prolonged release of 5-ASA from the sachet compared with the tablet. The Pentasa sachets contain mesalamine and also have a prolonged release; the drug is released in the terminal ileum and ascending colon.
Dosing of mesalamine
Dose response studies for mild to moderate active UC reported additional benefit of 2.4 g/d compared with 1.6 g/d, and another study showed added benefit of 4.8 g/d dosing compared with 1.6 g/d. Two meta-analyses recommended dosing 3 g/d or greater for mild to moderate active UC. The regimen commonly used by clinicians is to administer a 2.4 g/d dose for mild to moderate active UC, with an increase to a maximum of 4.8 g/d if patients are not responsive to the lower dose. The Assessing the Safety & Clinical Efficacy of New Dose of 5-ASA (ASCEND) trials involved 2 randomized, double-blind placebo-controlled trials comparing 4.8 g and 2.4 g/d dosing of mesalamine. The ASCEND I trial involved 301 patients with mild to moderately active UC with a primary end point of overall improvement (complete remission or clinical response to therapy) at week 6 of treatment in the format of a noninferiority trial. The results revealed significant improvement on the higher dose compared with the lower dose for those with moderately active UC (57% in 2.4 g/d, 72.4% in 4.8 g/d; P = .0384). The ASCEND II trial involved moderately active UC only (286 patients), and patients were randomized to the 2.4 g/d dose or 4.8 g/d daily dose of mesalamine; the primary end point was the same as that of the ASCEND I trial. Results yielded a significant treatment success in those on the 4.8 g/d dose (72%, 89/124 patients) compared with those on the 2.4 g/d dose (59%, 77/130 patients, P = .036). Adverse events were similar in both groups. The studies suggested that the higher dose is more effective at providing acute response and remission in patients with moderately active UC, while the lower dose is sufficient in patients with mildly active UC.
The notion that a higher dose (4.8 g of mesalamine) is superior to 2.4 g of mesalamine is not true in all populations studied. The primary end point of the ASCEND II and III studies was overall improvement at 6 weeks, which was determined by the Physician’s Global Assessment (PGA), which encompassed the clinical assessments of rectal bleeding, stool frequency, and sigmoidoscopy findings.
In ASCEND III, only patients with moderately active UC were studied. Overall, 70% of patients (n = 273/389) achieved overall improvement with Asacol HD at 4.8 g/d compared with 66% of patients (n = 251/383) who took Asacol 400 mg tablets at 2.4 g/d. (In ASCEND II, 72% of patients [n = 89/124] achieved overall improvement with Asacol HD at 4.8 g/d at 6 weeks, as compared with 59% of patients [n = 77/130] who took Asacol 400 mg tablets at 2.4 g/d [ P = .036]). Thus, when compared with each other, 2.4 g of mesalamine in the formulation of delayed-release mesalamine has similar efficacy as compared with 4.8 g of mesalamine for active treatment of ulcerative colitis. In a post hoc analysis there is, however, a suggestion that certain subsets of patients may benefit from high dose (4.8 g of mesalamine). In particular, in the ASCEND III trial there is a therapeutic advantage for the 4.8 g/d dose compared with the 2.4 g daily dose observed among patients previously treated with corticosteroids, oral mesalamines, rectal therapies, or multiple UC medications.
The convention that dosing 5-ASA compounds multiple times a day was challenged initially by studies of Kamm colleagues and Lichtenstein colleagues using MMX mesalamine in assessing efficacy of induction of response and remission. Similarly, a once daily formulation was found to be effective for maintenance of remission by using Apriso for maintenance of remission in 2 6-month maintenance studies.
The same findings were reproduced in a study evaluating delayed release mesalamine by Sandborn and colleagues. This multicenter, randomized, investigator-blinded, 12-month, active-control trial involved 1023 patients and compared once daily (QD) dosing to twice daily (BID) dosing of delayed-release mesalamine (Asacol) 1.6 to 2.4 g/d in patients with UC and who were currently in clinical remission (for at least 3 months). The primary end point was maintenance of clinical remission at 6 months. At month 6, 90.5% (428/483) of patients on the QD dose and 91.8% (435/474) on the BID dose maintained remission (95% confidence interval [CI] for BID-QD, −2.3, 4.9, respectively). The study was designed to be a noninferiority design trial. At month 12, 85.4% (379/444) or patients on the QD dose and 85.4% (380/445) of the BID dose maintained clinical remission (95% CI for BID-QD, −4.6, 4.7 respectively). Withdrawals due to adverse events were similar in both groups. The study concluded that once-daily dosing of Asacol at doses of 1.6 to 2.4 g/d was effective for the maintenance of clinical remission in patients with UC.
Foam versus liquid enema
Rectal administration of 5-ASA has been advocated as the treatment of choice in proctitis, proctosigmoiditis, and left-sided UC because local concentrations and active drug at the affected site are sufficiently high for therapeutic activity, and in addition, systemic absorption is considerably lower. Other advantages include a generally quicker response time and less frequent dosing schedule. Different meta-analyses of controlled trials have also indicated a superiority of topical mesalamine over oral 5-ASA alone in achieving clinical improvement in patients with mild to moderate distal colitis. However, in practice, rectal application often serves as an alternative or add-on therapy to oral 5-ASA.
Mesalamine rectal suppositories have been shown effective in the treatment of proctitis and maintenance of remission; however, their use is limited because they do not provide sufficient spread of the active ingredient beyond the distal colon. The spread of mesalamine liquid enema formulation is generally good, and has been shown to be effective in inducing and maintaining remission in distal colitis. However, patient acceptance may be compromised by difficulties with self-administration, retention, discomfort, or the necessity for prolonged bed rest. Rectal mesalamine foam was developed to optimize drug delivery and improve patient acceptance. The rectal foam has a higher viscosity than the liquid enema, which favors retention, enhances mucosal adhesion, and provides wider mucosal spread.
An internal pharmaceutical company report assessed the extent of dispersion of the rectal foam in a gamma scintigraphic study in healthy volunteers. It was found that immediately after dosing, the foam had spread through the rectum and sigmoid colon, reaching the upper descending colon after 12 hours.
Pokrotnieks and colleagues in a multicenter, randomized, double-blind parallel-group study showed that mesalamine foam was well tolerated and more effective than placebo in patients with distal UC. In this study, 111 patients with mild to moderately active proctitis, proctosigmoiditis, or left-sided UC received mesalamine foam or placebo enema for 6 weeks. Clinical remission was more frequent in the mesalamine than the placebo group (65% vs 40%; P = .0082). The frequency of endoscopic remission was also higher in the mesalamine group (57%) than in the placebo group (37%). In comparing the efficacy of mesalamine foam with mesalamine liquid enema, Cortot and colleagues conducted a multicenter investigator-blinded clinical trial and randomized 375 patients with mild to moderate UC to rectal foam or liquid enema for 4 weeks. Eligible patients were 18 years and older with newly diagnosed or relapsing active mild to moderate left-sided UC, with a disease extension of at least 5 cm from the anal margin and not above the splenic flexure. Remission rates at week 4 in mesalamine foam versus mesalamine liquid enema were 68.3% versus 73.6% in the per protocol (PP) population (lower limit of 97.5% CI −15.1%) and 66.7% versus 70.5% in the intention-to-treat (ITT) population (97.5% CI −13.4%). Remission rates at week 2 were 48.1% versus 50.6% in ITT (97.5% CI −12.8%) and 49.1% versus 52.1% in PP (97.5% CI −13.8%) in foam versus liquid enema, respectively. Both treatments were well tolerated with minimal adverse events. Although the noninferiority of mesalamine foam could not be strictly demonstrated at week 4 in the PP analysis, it was achieved in the ITT population and at week 2 in both populations. Mesalamine foam represents a clinically efficient and well tolerated therapeutic alternative to mesalamine liquid enema in patients with mild to moderately active proctitis and proctosigmoiditis. It may be especially appropriate in cases of poor tolerance of enemas because of acute rectal inflammation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







