Fig. 14.1
(a, b) Rare case of a huge palpable and visible renal mass in an 83-year-old man (it was a giant cystic tumour of the right kidney) (Courtesy of A. Benzaquin and A. Dominguez Beautell, Urology, Arnault-Tzanck Institute, Saint-Laurent-du-Var, France)
Constitutional symptoms like fatigue, loss of weight, fever and nausea occur in advanced disease.
An important and fortunately rare presentation is spontaneous retroperitoneal haemorrhage and spontaneous rupture of RCC. This surgical emergency presents with flank or abdominal pain, palpable mass, hypotension and haemorrhagic shock [6–8]. The cause of perirenal bleeding often remains unclear after CT scan and differentiation of RCC from AML remains difficult [9] until urgent life-saving nephrectomy is performed (Fig. 14.2).
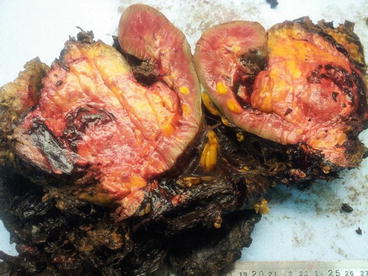

Fig. 14.2
Renal AML presented with acute retroperitoneal haemorrhage and shock (Courtesy of Sadiq Lala, Urology, the Royal hospital, Muscat, Oman)
14.2 Paraneoplastic Syndromes (PNS)
RCC is the most frequent urological malignancy associated with PNS [10] with 10–40 % of patients affected. PNS are a constellation of protean manifestations which include signs, symptoms and laboratory abnormalities that are not related to tumour local effect or metastatic disease. Generally, PNS indicate poor prognosis, but may be the presenting symptom in an early localized cancer which is still curable. PNS are caused by biologically active substances secreted by the tumour itself or by normal tissues as an immune response to the tumour. These substances include parathyroid-like hormone, erythropoietin, gonadotropins, renin, glucagon, insulin, human chorionic gonadotropin and ACTH-like substance [11]. Constitutional symptoms in RCC are often paraneoplastic manifestations mediated by cytokines. In up to one-third of patients fever, weight loss and fatigue are the first presenting symptoms. Among this the most common is cytokine-mediated neoplastic fever, which is found in 20–30 % of cases and is the sole presenting complaint in 2 % of patients. IL-6 has been identified as a possible pyrogen, and 78 % of patients with raised IL-6 level in RCC had fever [12, 13]. Fever in localized RCC subsides with nephrectomy, and may return with recurrence. Other cytokines include tumour-necrosis factor-α (TNF-α) which is known to alter adipocyte metabolism and affect appetite control, IL-1, certain interferons and prostaglandins [14].
The most important manifestations of PNS are as follows.
14.2.1 Hypercalcaemia
Reported in 10–20 % of patients with RCC, hypercalcaemia is an indicator of advanced disease and poor prognosis. Seventy-five per cent of patients with hypercalcaemia have high stage lesions. Albright first proposed PTH-like factors as the cause of this hypercalcaemia in 1941 [15]. From 1964, other researchers identified the PTH-related peptide (PTH-rP) as responsible for this phenomenon [16, 17] (Fig. 14.3). It should be noted that RCC produces both PTH and PTH-rP [18] and that PTH-rP is not unique to tumour cells. Its overproduction in malignant state is thought to be the result of altered gene expression [19].
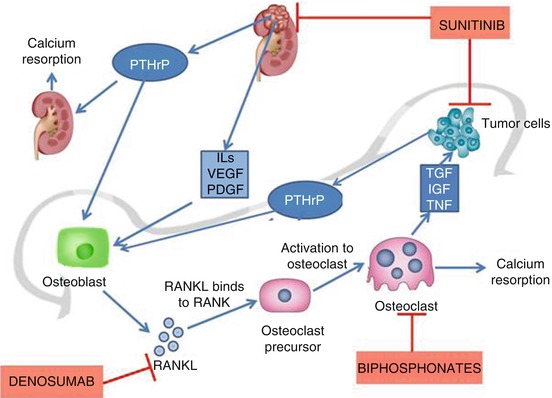

Fig. 14.3
Mechanism of hypercalcaemia in RCC. Vicious cycle of hypercalcaemia. Renal cell carcinoma secreting PTHrP causes renal reabsorption of calcium and stimulates osteoblasts. Osteoblasts produce RANKL which in turn binds to RANK receptor on the surface of osteoclast precursor, resulting in activation to osteoclasts. Osteoclasts cause bone resorption and produce tumour growth factors which enhance metastatic tumour growth. Denosumab is a monoclonal antibody that binds to RANK inhibiting the formation of osteoclasts
PTH-rP binds to PTH receptors in renal tubules and bone. This increases bone resorption of calcium, decreases renal clearance of calcium and increases phosphorus excretion. Also PTH-rP inhibits 1.25 hydroxylase in kidneys lowering the level of active vitamin D3. The net result is hypercalcaemia, reduced levels of PTH and Vit D3 and renal phosphate wasting. Other mediators are also known to potentiate this cascade, like TGF α and β, IL-1, IL-6 and TNF [20, 21].
Note: Hypercalcaemia secondary to bone metastasis accounts for 50 % of hypercalcaemia in RCC and is not a true PNS. Metastatic tumour in the bone liberates substances that activate osteoclasts causing the release of calcium from bone. Local secretion of prostaglandins and IL-6 were attributed to this reaction. Patients may have bone pain, which can be alleviated by radiotherapy. Medical therapy is beneficial in managing systemic effects. Agents such as biphosphonates and osteoprotegerin were proven effective in the treatment of lytic bone disease mediated by osteoclast activation [22].
Symptoms and signs of hypercalcaemia are systemic such as fatigue, lethargy, nausea, weakness, confusion, constipation, reduced deep tendon reflexes (DTRs) and impaired level of consciousness. Other signs include dehydration, ECG changes such as prolonged PR and QT interval, bradyarrhythmias and asystole. Patients presenting in hypercalcemic coma have also been reported [23]. Management includes initial hydration by intravenous infusion of normal saline and loop diuretics (furosemide), and avoidance of thiazide diuretics. Biphosphonates are effective in long-term management. Indomethacin (NSAID drug) has been reported as effective in patients with high serum prostaglandins. Nephrectomy was reported to be effective in relieving the associated hypercalcaemia [24, 25].
Treatment with sunitinib, tyrosine kinase inhibitor has been reported to successfully control paraneoplastic hypercalcaemia which was resistant to conventional therapy. Sunitinib also helps in alleviating symptoms [26].
14.2.2 Hypertension
Long-standing hypertension is a risk factor for RCC and evidence also suggests that RCC causes hypertension [27]. Hypertension is experienced in 40 % of patients with RCC as a PNS, whereas the incidence in age-matched control is 20 %. Cause is attributed to various peptides released from RCC, increased renin secretion (by the tumour itself or by the adjacent renal parenchyma due to tumour compression and hypoxia), presence of arteriovenous fistula, tumour compression of the renal artery or its branches and polycythaemia. Nephrectomy has been reported to reduce the hypertension in these patients. Renin elevation was found in up to 37 % of patients. It was correlated with high grade and stage of RCC with mixed histological subtypes and indicated poor prognosis. The role of renin as the cause of hypertension in RCC is controversial [28, 29]. Many renal epithelial neoplasms like oncocytoma and chromophobe carcinoma express renin in the cytoplasm of tumour cells. However, the role of this renin in hypertension is not clear, being structurally abnormal and biologically inactive, and its secretion into circulation is doubtful. Most of the RCC tumours are hypervascular and may harbour small arteriovenous fistulas (AVF) [30, 31]. Rarely tumour AVF produces sufficient left to right shunting to cause decompensation of the cardiovascular system, leading to intractable hypertension and congestive cardiac failure. Nephrectomy offers a dramatic improvement in hypertension and resolution of clinical cardiovascular symptoms.
14.2.3 Polycythaemia
Erythropoietin (EPO) is a glycoprotein normally produced by the peritubular interstitial cells of the kidney and functions to promote red cell production from bone marrow. RCC behaves as an ectopic site of EPO synthesis and raised EPO has been observed in 63 % of cases [32, 33]. Level of EPO in RCC was not shown to correlate directly with tumour grade, stage, histological type, prognosis or haematocrit and haemoglobin levels. Even though most of patients with RCC produce EPO, erythrocytosis and polycythaemia has been noted only in about 5 % of RCCs. Tumour cells may be producing inactive forms of EPO. In patients with organ-confined disease, EPO falls to normal after nephrectomy, but in mRCC, it tends to remain elevated. EPO can have a role as a marker during therapy. It has been reported recently that targeted presurgical sunitinib therapy in mRCC successfully controlled polycythaemia caused by EPO production in tumour cells [34].
14.2.4 Anaemia
Anaemia is more common in RCC than polycythaemia. Tumour cachexia is an important cause of the anaemia. Lactoferrin, a glycoprotein produced by RCC cells is another suggested cause [35]. Lower serum iron levels correlate with the RCC stage, the effects of surgery and the advent of recurrence [36].
14.2.5 Stauffer’s Syndrome
Maurice H. Stauffer, a gastroenterologist in Mayo clinic, first characterized the syndrome in 1961, with the original name ‘nephrogenic hepatomegaly’ [39]. This unique paraneoplastic syndrome associated with RCC, is a nonmetastatic hepatic dysfunction and comprises a constellation of signs and symptoms. It is characterized by an elevation of liver enzymes and abnormal levels of hepatic synthetic products and has been reported in 3–20 % of RCC patients. Clinically patients present with hepatosplenomegaly, fever and weight loss. There is a raised serum alkaline phosphate in all patients, an elevated prothrombin time or hypoalbumineamia in 66 %, an elevated serum bilirubin or transaminase levels in 20 % and an elevated gammaglobulin in 54 %.
Diagnosis requires at least three of these serum abnormalities. Thrombocytopenia and neutropenia are other findings.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







