Type
Percent
Subcutaneous
10–15
Low anal
60–70
High anal
15
Anorectal ischiorectal pelvic rectal
5
Submucosa, high intermuscular
Very rare
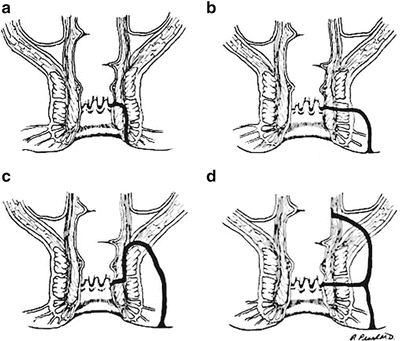
Fig. 7.1
The classification of anal fistulas. (a) Intersphincteric. (b) Transsphincteric. (c) Suprasphincteric. (d) Extrasphincteric (With permission Parks et al. [2])
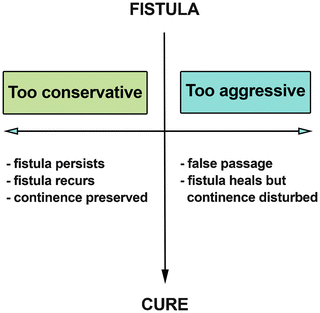
Fig. 7.2
Management goal in fistula surgery
The four main classes of fistulas are:
1.
Intersphincteric: The fistula originates at the dentate line and tracks caudad between the internal and external sphincter opening in the perianal region close to the anal verge. This fistula is seen following a perianal abscess and is typical of the ones seen in fistulizing midline anal fissures.
2.
Transsphincteric: The fistula originates at the dentate line and traverses the internal and external sphincter opening in the ischiorectal fossa. Depending on the height of the fistula varying degrees of sphincter involvement may be encountered. Treatment of high transsphincteric fistulas is more challenging due to increased risk of incontinence.
3.
Suprasphincteric: Originates at the dentate line and tracks cephalad to the external sphincter mechanism before opening into the skin at the ischiorectal fossa. These fistulas are not amenable to simple fistulotomy due to high risk of total incontinence
4.
Extrasphincter: Traverses the entire sphincter mechanism including puborectalis, opening proximally either at the dentate line (secondary to supralevator abscess) or in the lower rectal wall (secondary to internal or external penetrating trauma) and distally in the ischiorectal fossa or in the buttocks. This type of fistula is often secondary to trauma or Crohn’s disease.
Management Strategies in Fistula-in-Ano
The aim of surgery for anal fistula is to cure the patient with minimal or no sequela. If the surgeon is too conservative, the fistula may persist or recur after a short period of “healing” but the patient’s continence is preserved. On the other hand, if the surgeon is too aggressive, a false passage may be created, or the fistula may heal with varying degrees of disturbance of continence (Fig. 7.2). So to follow the dictum of “primum non nocere,” it takes an accurate assessment of the fistula and an experienced surgeon who deals with fistulas on a daily basis to perform the appropriate operation and prevent postsurgical incontinence (Fig. 7.3).
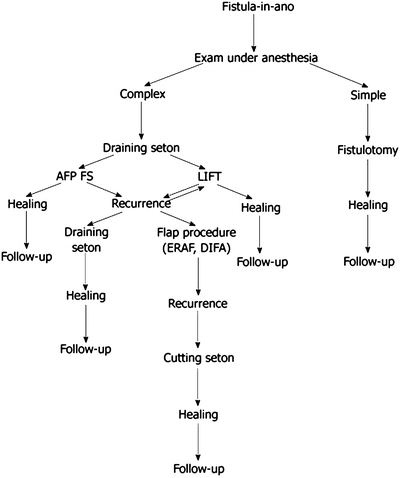

Fig. 7.3
Treatment algorithm for simple and complex fistula-in-ano
Fistulotomy or Fistulectomy
There has been and continues to be a basic controversy whether fistulotomy or fistulectomy should be performed for anal fistulas. There has been only one randomized trial comparing the two techniques by Kronborg [3] who alternately assigned patients to one or the other procedure. Fistulotomy fared better due to prolonged healing time associated with fistulectomy. A small section of the tract can be excised (biopsied) if there is concern for Crohn’s disease or malignancy, in cases of long-standing fistulas. Nelson and colleagues stressed the need for taking a biopsy from recurrent abscess wall or fistula tract to exclude malignancy. Low rectal or anal cancer (adenocarcinoma) may penetrate tissue and manifest as a recurrent abscess fistula. On the other hand, a very long-standing fistula in ano may develop into a squamous cell cancer [4]. The presence of mucus in fistulous abscess should raise the index of suspicion of malignancy [5].
Primary Fistulotomy
At the time of drainage of an abscess, the surgeon may find a fistula right away or after gentle probing with a blunt tipped probe. Primary fistulotomy was reported to be safe and with no adverse consequences in 1,000 consecutive cases [6]. Ramanujam et al. reported on 1,023 anorectal abscesses. Where the fistula was identified at the time of the incision and drainage and the experienced surgeon felt comfortable with primary fistulotomy, the results of primary fistulotomy were excellent and the recurrence rate was 3.4 % (Table 7.2) [7]. However, Vasilevsky and Gordon found fistulas in only a third of drained abscesses and argued against primary fistulotomy [8]. In any case primary fistulotomy requires an experienced surgeon exercising good judgment and extreme care in order to avoid postsurgical fecal incontinence and worse yet, recurrence due to creation of an inadvertent false passage.
Type of abscess | Number of abscesses | % of Fistulas |
|---|---|---|
Intersphincteric | 219 | 47.4 |
Suprasphincteric | 75 | 42.6 |
Intersphincteric | 437 | 24.5 |
Ischiorectal | 233 | 25.3 |
High Intermuscular | 59 | 15.2 |
Staged Fistulotomy implies that a high or complex fistula is treated in stages. At the first operation a portion of the sphincter mechanism is incised and a loose (marking) seton is placed around the remaining (undivided) external sphincter. After a period of 6–8 weeks the patient is reexamined under anesthesia and if the first stage sphincterotomy is healed by fibrosis, the remainder of sphincter is divided and the setons removed. In a study of 480 fistulotomies, Ramanujam and colleagues reported excellent healing with minimal (2 %) impairment of continence [9]. However; this report preceded the development of anorectal physiologic studies. In a larger study of staged fistulotomy using seton from the same institution Pearl and colleagues reported recurrence rate of 3 % and major incontinence, defined as the need to wear a pad, in 5 % of 110 patients treated in this fashion (Fig. 7.4) [10]. This is in contradistinction of the incidence of incontinence in cutting setons, which is reported to be as high as 12 % in a meta-analysis [11].
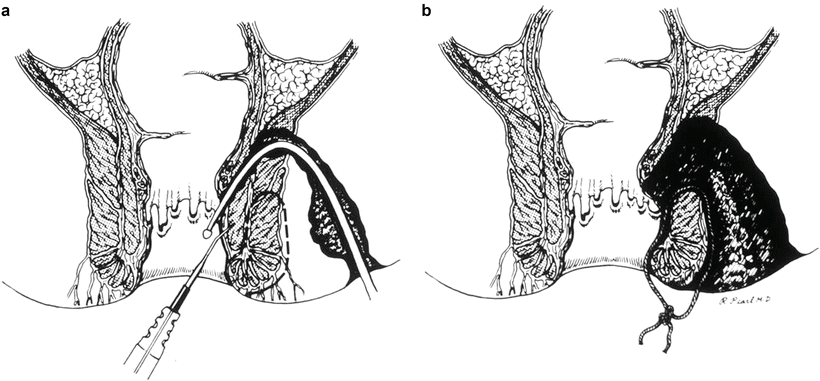

Fig. 7.4
Staged fistulotomy for transsphincteric fistulas. (a) Primary fistulotomy with seton (Courtesy of Dr. Russell Pearl). (b) Second fistulotomy (Courtesy of Dr. Russell Pearl)
Inpatient or Outpatient Fistulotomy
Outpatient fistulotomy is the standard of care for majority of fistulas. A straight local anesthesia, local anesthesia with conscious sedation or local anesthesia with monitored anesthesia care may be utilized depending on patient’s emotional state and tolerance of pain. Spinal and general anesthesia can be utilized for more complex cases needing longer operative times. Ambulatory surgery was also recommended by the Standard Task Force of the American Society of Colon and Rectal Surgeons [12].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








