NATURAL HISTORY OF CKD
■ Early: Usually asymptomatic in its early stages
■ Late: Symptoms and signs (usually related to sodium and water retention, manifesting as hypertension and edema) in association with metabolic and hormonal complications (anemia, vitamin D deficiency, secondary hyperparathyroidism) as well as an increased incidence of cardiovascular disease, infection, and impaired physical function
EVALUATION OF CKD
■ Exclude reversible prerenal factors (i.e., renal hypoperfusion): Hypovolemia, hypotension, infection, and administration of drugs that lower the GFR such as NSAIDs and renin–angiotensin system (RAS) blockers. Unlike the situation in patients with normal kidneys, a low fractional excretion of sodium (FENa) may not be present in CKD because of the decrease in filtered load of sodium (fractional excretion = amount excreted/amount filtered) (see Chapter 9).
■ Exclude postrenal factors (i.e., obstruction): Usually, this is easily done by ultrasound (bladder scanning and/or renal ultrasound). This is essential in older patients, particularly men. Ultrasound also gives additional helpful information, such as documentation of two kidneys, kidney size, evaluation for renal cysts or masses, exclusion of polycystic disease, identification of nephrocalcinosis or calculi, and, in some centers, evaluation of renal vasculature (see Chapters 9 and 17).
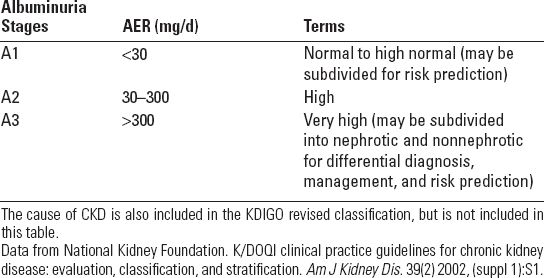
■ All patients with CKD should have a basic evaluation including complete blood count (CBC), urinalysis, electrolytes, renal function (blood urea nitrogen and creatinine), fasting glucose, calcium, phosphorus, magnesium, intact parathyroid hormone (PTH), 25-hydroxy-vitamin D, and urine albumin and total protein-to-creatinine ratio. Further evaluations will depend on initial findings and likely diagnostic possibilities. For instance, the presence of hematuria and proteinuria suggests glomerulonephritis (see Chapter 10) and work-up should include measurement of antinuclear antibodies, complements (C3, C4), hepatitis panel (B and C), and antineutrophil cytoplasmic antibodies. Nephrotic syndrome should prompt evaluation for diabetes (glycated hemoglobin) and consideration of primary AL amyloidosis (serum protein electrophoresis [SPEP], urine protein electrophoresis [UPEP] serum and urine free light chains) and HIV-associated nephropathy (HIVAN) (HIV antibodies, viral load). A high urine total protein without albuminuria suggests paraproteinuria due to myeloma or primary AL (SPEP, UPEP, serum and urine free light chains).
■ Determination of rate of CKD progression: Untreated CKD will typically lead to a progressive decline in GFR at a characteristic rate, depending on the etiology of the disease, the degree of proteinuria, blood pressure control, and other factors. A rate of decline in GFR of >5 mL/min/1.73 m2/y is considered to be rapid progression; in most patients, GFR will decline at a rate of 2 to 4 mL/min/1.73 m2/y), though some patients will progress at a very slow rate (<1 mL/min/1.73 m2/y), a rate not distinguishable from the normal age-related decline in kidney function. In an individual patient, the rate of GFR decline in the past is a very good predictor of the future course of kidney disease and whether to expect end-stage kidney disease (ESKD) to develop. For instance, a 55-year-old patient with a GFR of 30 mL/min/1.73 m2 and a rate of GFR decline of 10 mL/min/1.73 m2/y despite aggressive management (see below) is almost certain to require dialysis or transplantation in the future (probably in about 2 years), but a 80-year-old patient with a GFR of 20 mL/min/1.73 m2 and a rate of GFR decline of 1 mL/min/1.73 m2/y may not live long enough to ever need dialysis.
MANAGEMENT OF CKD
■ Nutrition: Before the availability of dialysis, the only therapy for uremia was to reduce protein intake. Although it is clear that protein restriction can decrease uremic symptoms, there is no clear evidence that it can reduce the rate of progression of CKD. However, moderate protein restriction (not less than 0.8 g/kg/d) may be useful and not detrimental. Phosphate restriction can prevent secondary hyperparathyroidism (see below) and may decrease vascular calcification, although this is still an unproven hypothesis. In the presence of hypertension or edema, sodium restriction is indicated (see below), and potassium restriction is necessary if there is hyperkalemia. The National Kidney Foundation (US) provides useful dietary guidelines (www.kidney.org).
■ Salt and water retention: As GFR declines, the ability of the kidneys to excrete dietary sodium is impaired, leading to a tendency to sodium retention (edema) and development of hypertension. Loop diuretics such as furosemide (Lasix) rather than thiazide diuretics are usually required, in particular when GFR is below 50% of normal. Occasionally, a combination of a loop diuretic and a thiazide-type diuretic (such as hydrochlorothiazide or metolazone) is needed. A brisk diuresis may ensue due to blockade of sodium transport at both the loop and distal tubule (see Chapter 14).
■ Hypertension: Edema in a hypertensive patient necessitates dietary salt restriction and institution of or increase in diuretics. Control of blood pressure in patients with CKD almost always requires diuretic therapy in addition to other antihypertensive agents, as most patients with CKD and hypertension will be volume overloaded even if edema is absent. Renin angiotensin system inhibitors are preferred in proteinuric CKD due to their proven renoprotective effects, though it is difficult, if not impossible, to determine if their beneficial effects are solely due to lowering of blood pressure or involve mechanisms specific to RAS inhibition. Moreover, hyperkalemia due to decreased aldosterone production or effect is a major problem in many patients. Dihydropyridine calcium blockers can increase proteinuria and glomerular injury and should be avoided in proteinuric CKD if possible. In the absence of proteinuria, there is no specific preferred or nonpreferred agent. Control of hypertension regardless of the drugs used is the most important intervention to slow progression of CKD (indeed, the only proven intervention). Other modalities that may be beneficial include control of blood glucose (in diabetics), lipid (statin therapy), and smoking cessation. Whether or not they affect CKD progression, these interventions are indicated for cardiovascular risk reduction (see Chapter 14).
■ Cardiovascular disease: Patients with CKD and especially ESKD have a high rate of cardiovascular disease, which has not been completely explained. Among the traditional risk factors, hypertension, diabetes mellitus, and hyperlipidemia are common in this population. Patients with CKD are also frequently obese with low physical activity. Nontraditional risk factors include proteinuria, oxidative stress, inflammation, hyperphosphatemia (leading to vascular calcification), and possibly anemia (although correction of anemia has not been shown to decrease cardiac risk) (Castaneda et al., 2004; Heiwe & Jacobson, 2011).
■ Anemia:
■ Pathogenesis: As GFR declines, erythropoietin (EPO) production by the kidney is impaired, which typically results in anemia (hemoglobin level <13.0 g per dL in males or <12.0 g per dL in females). Anemia is generally present with moderate CKD, though severe anemia (hemoglobin <9 g per dL) is unusual unless CKD is severe. Other factors, in particular iron deficiency, should be excluded (McGonigle et al., 1984).
■ Symptoms: The onset of anemia in CKD is gradual and patients may not be symptomatic. If present, symptoms may include fatigue, weakness, and lack of energy and enthusiasm. Examination may show pallor. If severe, symptoms of heart failure may occur.
■ Laboratory evaluation:
• CBC—confirms anemia and may suggest an alternative diagnosis other than anemia of CKD (e.g., if there is pancytopenia, schistocytes on the peripheral smear, etc.)
• Serum iron/total iron-binding capacity (TIBC) ratio (transferrin saturation or TSAT) and ferritin—iron and ferritin are low and TIBC is elevated in iron deficiency. Ferritin is an acute phase reactant and is increased in acute or chronic illness.
• Vitamin B12 and folate—may be deficient and should be investigated.
• Reticulocyte count—is a measure of bone marrow response
• Hemoccult—gastrointestinal bleed is a common confounder
• Haptoglobin, Coombs—exclude hemolysis
• EPO levels are generally not necessary unless degree of anemia is out of proportion to degree of CKD.
•
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


