Risk factor
Absolute risk
RRa
Ref
Disease duration
At 10 years: 2–3 %
2.4
At 20 years: 8 %
2.8
At 30 years: 18 %
Extent:
Ulcerative pancolitis
14.8
[3]
Left-sided UC
2.8
Ulcerative proctitis
1.7
Presence of PSCb
At 10 years: 9 %
4.8
[10]
At 20 years: 31 %
At 25 years: 50 %
[11]
CRC in first-degree relative (FDR)
>50-year-old FDR
2.5
<50-year-old FDR
9.2
[10]
Disease onset at
Age <15 years
40 %
Age 15–39 years
25 %
[8]
11.3 Colonoscopic Surveillance for CRC Risk in Long-Standing Inflammatory Bowel Disease
Surveillance Protocol. Surveillance colonoscopy (using WLI) is best performed in stable remission of colitis (Truelove activity index ≤2) [12] and combined with CE using indigo carmine or methylene blue for targeted biopsy strategy [4]. After 8 years of UC, screening and further surveillance colonoscopy is recommended with targeted biopsies of suspicious areas from each anatomic section or quadrant biopsies every 10 cm from the involved colon. Analogous recommendations pertain to Crohn’s colitis when at least 30 % of the colon are involved [4]. This typically results in 28–32 biopsy samples at a minimum. The procedure report in ulcerative colitis or Crohn’s colitis should number the locations of all biopsies sampled from flat mucosa, from any mass (image documented) or any suspicious polypoid lesion sampled or removed.
Chromoendoscopy and Magnifying NBI. In well-cleaned, mucus-depleted large bowel, IEE using panchromoendoscopy with indigo carmin or methylene blue should be employed to take targeted biopsies. Panchromoendoscopy and targeted biopsies more recently resulted in a higher yield of dysplasia than systematic 4-quadrant biopsies in non-dye sprayed colon [13–15]. Virtual chromoendoscopy using NBI has not shown to be of improved benefit identifying flat dysplasia.
However, optimal high-definition WLI proved comparable sensitivity and specificity as NBI for detection of dysplastic lesions (DALM and flat dysplasia) in UC – but the sensitivity of both pit pattern (PP) and microvascular pattern (CP) was in the range of only 76–80 % for differential diagnosis of neoplastic versus regenerative alterations [16]. Beyond endoscopic techniques, you must be aware of additional factors that influence the success of surveillance colonoscopy in IBD [4]:
Endoscopic detection of neoplasia
Adequate mucosal sampling
Low interference by strictures or pseudopolyps
Completeness of endoscopic resection of ALM
Patient compliance
Lesions in IBD. To detect areas suspicious for neoplastic lesions in regenerative chronic inflammatory mucosa, you must focus on subtle alterations in microsurface structure (PP III – V [17]) and CP [18] (compare Chaps. 4 and 10) as well as on visible surface alterations likely to harbor premalignant/malignant tissue [4, 17] (Table 11.2). Nevertheless, previous intensity of inflammatory activity correlates positively with an increased risk of HGIN or cancer. By analogy, the presence of postinflammatory pseudopolyps approximately doubles the risk of HGIN or cancer in UC [4, 19]. Long-standing UC with stricture or foreshortened colon has a high probability of even advanced cancer [20].
Adenoma-like ALM(→ endoscopic resection) | Non-adenoma-like DALMa (→ endoscopically non-resectable) |
|---|---|
Sessile/pedunculated | Usually sessile (broad based) |
Well circumscribed | Poorly circumscribed |
Smooth surface | Irregular surface |
Visible margins | Indistinct margins |
Non-ulcerated | Ulceration/necrosis |
No stricture | Stricture |
No mucosal tether | Mucosal tethering |
Note
Be observant for the principal premalignant/malignant lesions in IBD:
Sporadic adenoma/dysplasia (outside IBD-involved colon)
Adenoma-like lesion or mass (ALM)
Raised dysplasia or dysplasia-associated lesion or mass (DALM)
Flat dysplasia
Sporadic adenomas in uninvolved parts of the colon in UC (or Crohn’s colitis) carry a low risk (<5 %) of associated dysplasia or CRC, as well as protruding “ALMs” in non-dysplastic mucosa of IBD-involved colon [21]. Both neoplasias are indications for complete endoscopic resection [4]. In most instances, flat and protruding adenoma-like ALMs exhibit a clear margin and tend to be more reddish (due to enhanced CP) than the surrounding inflamed mucosa or – in remission – fibrous-atrophic mucosa (Fig. 11.1). Non-dysplastic regenerative colonic mucosa shows pit pattern type II or I, rarely similarity with PP type IIIL or IV and augmented regular CP – but small regenerative areas never exhibit a lesion with clear margins [22] (compare Fig. 11.2). For morphology and endoscopic analysis of neoplastic lesions in colorectum, refer to Chap. 10.
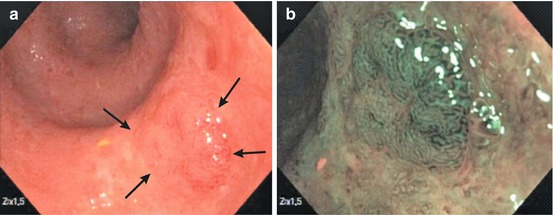
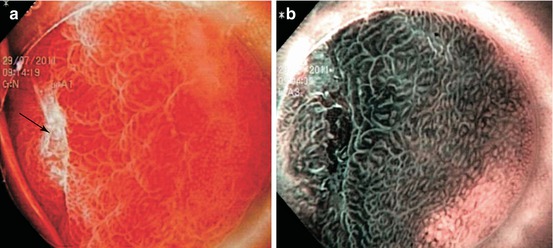

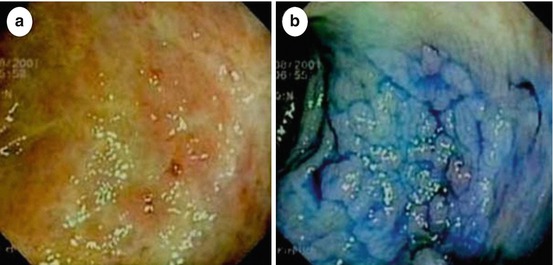
Fig. 11.1
(a) Chronic moderately active ulcerative colitis with a lesion type 0-IIa, of 15 mm diameter with distinct margins (arrows). Mid-sigmoid colon (69-year-old man). WLI (zoom 1.5-fold). (b) Same lesion on NBI (zoom 1.5-fold) observation presenting PP IIIL and MVP II with distinct margins. ESD en bloc revealed tubular adenoma with LGIN

Fig. 11.2
Regenerative mucosa after healing of ESD ulcer in cecum, like red ulcer scar. (a) Flat lesion (like 0-IIb + IIc) of red ulcer scar with tiny central ulcer (→), 8 weeks after cecal ESD (WLI, 50× magnification). Red lesion with uncertain margin and gradual transition from PP-I (slit-like pits) to PP-II with “cobble-stone surface relief” and MVP-II. Typical red ulcer scar of healing ulcer. (b) NBI Zoom 40× (same lesion as Fig. 11.2a), Regenerative hyperplasia in the center (similar to PP III-L) and an uncertain margin to the periphery with PP type I
DALM are raised dysplastic lesions (0-IIa or 0-Is) with concomitant dysplasia of the surrounding flat mucosa (showing pit pattern IIIs, IIIL, IV, [V]) – also termed “non-adenoma-like DALM” (Figs. 11.3c–f and 11.4). This appears to be a “field cancerization defect” [4, 23] and has a high probability (38–84 %) of synchronous or metachronous cancer in chronic UC or Crohn’s colitis [4]. Endoscopic differential diagnosis between neoplastic and regenerative raised lesions (Fig. 11.3a, b) is very difficult in many cases of long-standing ulcerative pancolitis and in presence of multiple polyps (compare Sect. 11.6 case 4).
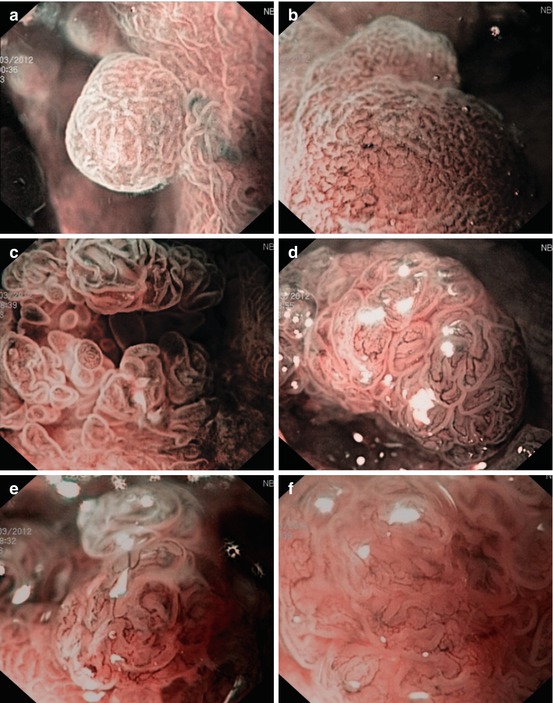
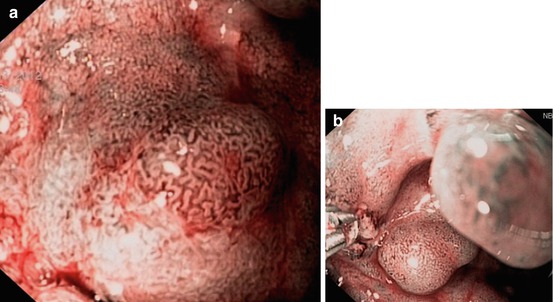

Fig. 11.3
(a–f) CP and PP of 0-Is lesions in long-standing ulcerative regenerative pancolitis with pseudopolyps (a, b) as well as DALM (c–f, M-NBI, 100×): (a, b) CP I meshed, and PP II, regenerative pseudopolyp. (c) CP II (dense) and PP III-L in rectal LST-GM (0-IIa + Is): Tubulovillous adenoma. (d) CP II (meshed) and CP IIIA (irregular, central part), PP IIIL in sigmoid lesion 0-Is. Histology: Tubulovillous adenoma with LGIN. (e, f) CP IIIA and surface pattern sparse to absent (PP Vi) in rectal lesion 0-Is. Histology: Adenoma with HGIN (differentiated mucosal cancer T0 m1)

Fig. 11.4
Non-adenoma-like DALM in case of multiple dysplastic lesions (compare case 2). (a) Dysplasia-associated lesion of mass (DALM) type 0-IIa with PP IIIL (center) and neighboring PP IIIs (left upper quadrant), mucin covered in lower part – in descending colon of a 51-year-old man with long-standing ulcerative pancolitis and multiple high-grade dysplasia-associated lesions or masses. (b) Biopsy in PP IIIs area revealed HGIN
Flat dysplasias (HGD) are similar to lesions type 0-IIb or IIc, sometimes nearly unrecognizable in chronic inflamed mucosa (Fig. 11.5). In case of high-grade dysplasia (HGD), cancer may already be present in 42–67 % of patients [24, 25]. By contrast, LGD may only carry a 3 % initial risk of concomitant CRC and a 10 % subsequent rate of progression to CRC within 10 years [26]. Gross features for ALM or DALM are listed in Table 11.2.