Early (<6 months)
Late (>6 months)
Extrahepatic:
Extrahepatic:
Stricture—single (anastomotic, external compression)
Benign stricture—single (anastomotic)
Stricture—multiple (HAT/ischemic)
Multiple (HAT/ischemic, rPSC)
Biliary leak
Stones
Cholangitis
Intrahepatic:
Intrahepatic:
Preservation/reperfusion injury
Acute cellular rejection
ABO incompatibility
Chronic rejection
HAT
Drug induced
Small-for-size graft
Recurrent disease (PBC, PSC, viral)
Drug induced
De novo viral hepatitis, autoimmune disease
Acute cellular rejection
Sepsis
Infection—CMV, bacterial, fungal, Hepatitis BCRC or HCV/HBV
Biliary Complications
Biliary complications following liver transplant occur in 10–50 % of transplants with associated mortality rates of 0–20 %, anastomosis revision rates of 12–25 %, and re-transplantation rates of 6–12 % [1–4]. The spectrum of biliary tract complications in the post-transplant setting may include biliary leaks, strictures, choledocholithiasis, cast formation, and other less commonly seen complications such as papillary stenosis [1]. Higher rates of biliary complications after a Roux-en-Y anastomosis are thought to be representative of its more complicated procedures (i.e., re-transplantation and diffuse extrahepatic diseases such as primary sclerosing cholangitis [PSC]) [3].
The most common biliary complications in the early postoperative setting include biliary leak and biliary obstruction related to both anastomotic and nonanastomotic strictures, with secondary cholangitis as a possible consequence. Biliary leaks can occur at the anastomosis, as a result of ischemic bile duct injury, or from the cut surface of the liver, particularly after a living donor or split liver transplant. The presence of bile in the output of operative drains is an early indicator of a bile leak, which can be confirmed with further testing radiographically or endoscopically. The benefit and use of T-tubes in reducing these complications are still debated [1–4].
Early intervention in biliary complications is important to graft survival. Biliary leaks or biliary strictures can often be treated nonsurgically. Endoscopic and percutaneous biliary stenting for strictures has the highest success rate (60–75 %) when biliary stents are placed following dilatation, as opposed to dilatation alone [5]. These procedures are most successful in early strictures (<6 months) rather than late strictures (>6 months) [3]. Traditionally, endoscopic biliary access was not possible in patients with Roux-en-Y hepaticojejunostomy, but advances in endoscopy technology—such as single- and double-balloon enteroscopy and overtube-assisted enteroscopy—have increasingly allowed for successful endoscopic diagnosis and treatment of biliary complications in these patients [1].
Nonanastomotic strictures occur at a frequency of 2–20 % [6]. They tend to be multifocal, can be both intrahepatic and extrahepatic, and are more likely to require re-transplantation. They can occur early (<6 months) or late (>6 months) and are associated with several underlying causes, including hepatic artery thrombosis (HAT), warm and cold/ischemic injury, ABO incompatibility, recurrent PSC, and possible viral infections such as cytomegalovirus (CMV) or even hepatitis C (HCV). These are discussed in more detail in later sections [6, 7]. Graft survival appears to be significantly lower in patients with nonanastomotic strictures when compared to matched patients without strictures [6]. Other important donor factors in patients receiving cadaveric organs may influence the development of biliary strictures [8–12].
Traditionally, cadaveric organs for liver transplants were donated after brain death (DBD). However, there are not sufficient numbers of these donors for patients currently waiting on liver transplant lists. Donation after cardiac death (DCD) is recognized as an important source of organs to increase the availability of donors for liver transplant. In DCD donors, declaration of death is based on cardiopulmonary criteria rather than cessation of brain and brainstem function and these organs are harvested after cardiopulmonary arrest in contrast to organs procured after established brain death. The reported outcomes of vascular (hepatic artery thrombosis) and biliary complications in DCD donors are more frequent than in DBD donors, resulting in an increased risk of graft loss [13]. In particular, these grafts appear to have a higher incidence of diffuse ischemic biliary strictures and cholangiopathy (IC).
The etiology of these higher rates of stricture seems to be related to increased donor warm ischemic times [14]. Other relevant operative features, such as the time from asystole to cross clamp, may also predict the development of biliary strictures [15]. As donor procurement techniques improve, the rate of biliary stricturing in these grafts will undoubtedly improve. While the rate of graft loss is higher in DCD than in DBD donors, the presence of biliary ischemic cholangiopathy does not necessarily result in graft loss. Many of these patients can be managed endoscopically in the early postoperative period with resolution of their biliary complications. It is, however, still too early to know the long-term complications and survival of these grafts.
Impacted biliary stones generally occur later post-transplant and are often associated with biliary strictures and impaired bile flow. Medications including cyclosporine may increase the risk of stone formation in the transplant setting [16]. These biliary stones can respond to endoscopic treatment [1].
Preservation/Reperfusion Injury and ABO Incompatibility
Preservation injury has been reported from approximately 17 % of transplants to as high as 50 % of transplants [17, 18]. Extrahepatic biliary complications, such as nonanastomotic strictures, occur with increasing frequency as the cold ischemia time reaches over 10–12 h [17, 18]. Preservation injury also occurs at the cellular level, involving nonparenchymal cells (sinusoidal endothelial cell injury and Kupffer cell activation with accumulating inflammatory cells and platelets) as well as parenchymal cells, including biliary epithelial cells [19, 20]. Histological findings of preservation/reperfusion injury include neutrophilic infiltration, microvesicular steatosis, hepatocyte cytoaggregation occurring early and progressing to centrilobular necrosis, hepatocyte swelling, and cholestasis later in the process [18]. Mild cases resolve spontaneously, but more severe cases may cause residual damage or result in primary graft nonfunction [18].
Blood group-related antigens (ABO) are expressed on the epithelial cells of large bile ducts and periductular hepatocytes [21]. Thus, the hepatic allograft may be more susceptible to immunologic bile duct injury after transplantation across the ABO barrier. Complication rates for biliary strictures, HAT, and cellular rejection were significantly higher in these patients [7]. Graft survival and patient survival have been inferior to ABO-matched transplants in retrospective studies [7].
Small-for-Size Syndrome
Small-for-size syndrome (SFSS) is a recognized complication occurring when the recipient receives inadequate functional tissue. It is thought to occur at a graft-to-recipient body weight ratio less than 0.8. The presentation can vary from mild isolated hyperbilirubinemia to graft failure. Within the first week of transplant the patient may develop cholestasis, prolonged coagulation, ascites, and variceal bleeding [22]. Histologic examination shows bilirubin plugs and cholestasis with patchy areas of necrosis and regenerative tissue [22]. The physiologic mechanisms of SFSS are not clear but seem to be related to portal hyperperfusion and arterial hypoperfusion resulting in endothelial disruption and subsequent molecular derangements on the hepatic regeneration process [22].
Hepatic Artery Thrombosis (HAT)
HAT is described in 3–9 % of transplants, with another 3 % of patients experiencing nonthrombotic complications such as hepatic artery stenosis, redundancy, and pseudoaneurysm [23]. In the transplanted liver, HAT results in more significant biliary damage than in a non-transplanted liver because the surgical excision interrupts smaller peripheral arteries that normally supply collateral flow [23]. One-third of HAT cases occurs within the first month and may present with cholestasis [24]. Because the main blood supply to the biliary system stems from the hepatic artery, later complications tend to include bile duct stricturing with subsequent cholangitis, liver abscess, biloma, and/or biliary necrosis with bile leak. Both early and late presentation of HAT can progress to severe liver failure [24, 25]. Re-transplantation rates of 50–80 % and mortality rates of up to 50–70 % have been described in patients with HAT [24–26].
Risk factors of HAT include vascular surgical technique, biliary anastomosis (Roux-en-Y), biliary leaks, cold and warm ischemic injury, antibody cross-match status, coagulation abnormalities, infections (particularly CMV), and possible immunologic factors [25, 26]. An increased risk has been associated with cigarette smoking in both the pre-transplant setting and the post-transplant setting [27]. Interventional radiology procedures (such as angioplasty and fibrinolysis) or immediate surgical revascularization are often needed to avoid re-transplantation [25, 28].
Infectious Complications
Bacterial and Fungal
Cholestasis with or without hyperbilirubinemia is frequently associated with extrahepatic bacterial or fungal infections and sepsis [29]. Evidence suggests that proinflammatory cytokines and endotoxin release are potent inhibitors of hepatobiliary transporter gene expression, resulting in hyperbilirubinemia and cholestasis [30]. Although seldom necessary, histological assessment may reveal nonspecific cholestasis and hepatitis or a neutrophilic infiltration of the bile ducts, biliary proliferation, and bile plugs termed “cholangitis lenta” [31].
Viral
Cytomegalovirus (CMV) is a common viral pathogen in liver transplant recipients and is reported in 30–80 % of transplant patients, with up to 30 % of patients developing CMV disease and 12–17 % CMV hepatitis. The highest risk is within the first 3–4 months post-transplant [29, 32]. CMV can infect and injure not only hepatocytes but also biliary epithelium and vascular endothelium [33, 34]. Thus, it can present as cholestasis. Histologic findings can range from lymphocytic infiltration, ballooned hepatocytes, cholangitis, cholestasis, endothelitis, and Kupffer cell reaction to more specific findings of microabscesses or, rarely, viral inclusions [35]. CMV has been implicated in vanishing bile duct syndrome and both acute and chronic rejection [33, 35].
Human herpesvirus-6 and -7 (HHV-6 and -7) activations are common and are usually associated with CMV infection and rejection in liver transplant patients [36]. They may present with cholestasis in a manner similar to CMV. Herpes simplex virus (HSV), as well as toxoplasmosis, should be considered with the immunosuppressed patient in appropriate settings [29]. Hepatitis C and hepatitis B viral (HBV) infections are discussed below.
Drug Induced
Drugs and toxins are common causes of cholestasis and can be the result of either a direct injury to the cells or an idiosyncratic reaction to a drug. Many of the commonly used drugs following transplant should be considered in the work-up of cholestasis. These drugs include immunosuppressive drugs, antibiotics, and antifungal agent. Table 10.2 outlines some of the more common post-transplant drugs that can contribute to cholestasis. In this chapter, we focus on a review of immunosuppressive medications.
Table 10.2
Common causes of drug-induced cholestasis after liver transplantation
Immunosuppression | Other |
|---|---|
Cyclosporine | Verapamil |
Tacrolimus | Seizure medications |
Sirolimus | Tricyclic antidepressants |
Everolimus | Oral glycemic agents |
Azathioprine | Hydrochlorothiazides |
Antibiotics: | |
Sulfa (trimethoprim/sulfamethoxazole) | |
Cephalosporins | |
Amoxicillin-clavulanate | |
Macrolide antibiotics | |
Isoniazid | |
Antifungal agents: | |
Fluconazole | |
Itraconazole |
Cyclosporine induces cholestasis by decreasing both bile flow and bile salt secretion. This suppression of bile salt synthesis reduces the bile salt pool size [37]. The drug inhibits bile salt and phospholipid secretion without a corresponding change in cholesterol secretion, thus elevating cholesterol saturation in bile and raising the potential risk for stone formation [16, 37]. In contrast to cyclosporine, tacrolimus shows higher bile flow rates and more rapid recovery of bile flow post-transplantation [38]. Cholestasis has also been attributed to tacrolimus, which may be the result of deranged bile acid transport or of impaired glutathione and bicarbonate excretion [38, 39]. Sirolimus and everolimus also have cholestatic effects through the retention of toxic metabolites within the hepatocytes [39, 40]. Azathioprine can cause hepatotoxicity of a variety of mechanisms, including cholestasis [41].
Acute Cellular Rejection and Chronic Rejection (CR)
The mechanism by which patients with acute cellular rejection (ACR) develop cholestasis is multifactorial. It is related to lymphocytic destruction of the small intrahepatic bile ducts or apoptosis of the biliary epithelial cell, and to impaired bile secretions related to Kupffer cell-induced cytokine release [42–44]. If early ACR is not treated or responds inadequately to treatment, progression to irreversible injury or chronic rejection occurs.
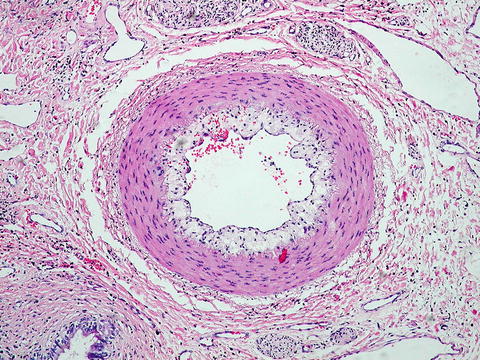
Chronic “ductopenic” rejection occurs in 2–5 % of patients and generally presents as cholestasis within 6 weeks to 12 months of transplantation. However, it has been noted as early as 2–3 weeks post-operation [45, 46]. It is characterized by progressive loss of intrahepatic, interlobular, and septal bile ducts (ductopenia) with lipid-laden vasculopathy in 50 % of portal tracts (Fig. 10.1) [46]. Risk factors for the development of chronic rejection include CMV infection and pre-transplant disease etiology. Immune-related diseases such as PSC, primary biliary cirrhosis [PBC], and autoimmune hepatitis [AIH] have the highest incidence. Human leukocyte antigen (HLA) mismatching, donor male to recipient female matching, and the number and/or severity of acute rejection episodes have also been linked to chronic rejection [34, 45]. Whether the pathogenesis is related to an immune-mediated injury or to an ischemic injury resulting from the obliterative arteritis is unclear [23, 47]. Undoubtedly the processes are linked, and it is the combination and severity of each component that results in the clinical manifestation of chronic ductopenic rejection.