Risk factors for intrahepatic cholangiocarcinoma
Viral hepatitis (B and C)
Fibrocystic diseases
• Autosomal dominant polycystic kidney and liver disease
• Choledochal cyst
• Caroli’s disease
• Congenital hepatic fibrosis
Chronic cholestatic diseases
• Primary sclerosing cholangitis
Parasitic infection
• Clonorchiasis sinensis
• Opisthorchiasis viverrini
Recurrent pyogenic (oriental) cholangitis and intrahepatic lithiasis
Cirrhosis
Gallstones
Extrahepatic biliary atresia
Inherited metabolic disorders
• Alpha-1 antitrypsin deficiency
• Hereditary hemochromatosis
Drugs/toxins
• Thorotrast
• Anabolic steroids (isolated reports)
Cigarette smoking
Diabetes mellitus
Alcohol abuse
Obesity (less established)
Intrahepatic cholangiocarcinoma most often presents with nonspecific symptoms such as abdominal pain, weight loss, cachexia, night sweats, and fatigue [6, 17]. The lack of specific symptoms can delay tumor detection, resulting in advanced stage tumors at the time of diagnosis. Imaging techniques, including computed tomography (CT) and magnetic resonance imaging (MRI), greatly assist in the diagnosis; radiologic features of intrahepatic cholangiocarcinoma generally differ from those of hepatocellular carcinoma [18]. However, the diagnosis most often requires biopsy confirmation. Imaging techniques are also essential in staging and predicting resectability [19].
10.1.3 Serum Markers
Several tumor serum markers have been proposed to detect and monitor intrahepatic cholangiocarcinoma [20]. Although useful in some cases, these markers suffer from moderate sensitivity and specificity, which limits their utility [21, 22]. Sensitivities and specificities, however, can be improved by combining two or more markers. Carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) are perhaps the most widely utilized in clinical practice. CA 19-9 is a sialylated Lewis blood group antigen targeted by the monoclonal antibody 116 NS 19-9 and is the most established serum marker for the diagnosis of intrahepatic cholangiocarcinoma. Studies have shown sensitivities and specificities ranging from 50 to 90 % and 54 to 98 %, respectively [23–25]. An elevated CA 19-9 has a positive predictive value for intrahepatic cholangiocarcinoma of only 57 % and is limited by both its occurrence mainly in advanced tumors and by its false positivity in benign biliary tract disease and cholangitis [20, 25]. According to some studies, however, elevated CA 19-9 may have prognostic utility (poor prognostic indicator) [26, 27]. Serum CYFRA 21-1 is another potential serum marker of intrahepatic cholangiocarcinoma that was developed to measure a soluble fragment of cytokeratin 19 in the serum. Serum CYFRA 21-1 has higher specificity than CA 19-9 for intrahepatic cholangiocarcinoma, but data remains limited [28–30]. This marker may be useful to detect and monitor intrahepatic cholangiocarcinoma, but has not been adopted for routine clinical use. CEA, another serum marker of intrahepatic cholangiocarcinoma, is inferior in sensitivity and specificity to CA 19-9 and can be expressed in a variety of carcinomas including gastric, colonic, and pancreatic carcinomas [20]. In contrast to CA 19-9, elevated CEA levels do not predict patient prognosis [31]. CA 125 and Sialic acid have also been studied as serum markers for intrahepatic cholangiocarcinoma, but are limited by a lack of specificity. Several other serum markers have also been described, including TuM2-PK and MMP-7 [20], but none are currently used in clinical practice.
10.1.4 Pathogenesis
Chronic inflammation, cholestasis (bile acids), and genetic and epigenetic alterations including mutagenesis, oncogenic pathway activation, evasion of apoptosis, and neoangiogenesis are involved in the pathogenesis of intrahepatic cholangiocarcinoma. Although a variety of chromosomal and genetic alterations are common in intrahepatic cholangiocarcinoma (e.g., k-ras, p53, MET, Bax, SMAD4, p16, IDH1/IDH2), many of which have prognostic implications, none are specific or diagnostically useful [32–34]. Some promising molecular alterations (such as FGFR2 rearrangements), nevertheless, may offer therapeutic targets [35].
Precancerous conditions associated with intrahepatic cholangiocarcinoma, including biliary intraepithelial neoplasia (BilIN) and intraductal papillary biliary neoplasms, are discussed in detail in Chap. 9.
10.1.5 Natural History/Prognosis
Because intrahepatic cholangiocarcinomas are often asymptomatic, they frequently come to clinical attention at advanced stage, resulting in an overall poor outcome; the overall 5-year survival rate is approximately 34 %, with a median survival time of approximately 2 years [36]. The only potential cure for intrahepatic cholangiocarcinoma is surgical resection for low stage tumors. Following surgical resection with curative intent, the overall 5-year survival is 63 % and the median survival is 36 months [2].
Metastases occur in 73 % of cases, most frequently involving lymph nodes and lungs, followed by peritoneum, adrenals, kidneys, and bone [7]. Histologic parameters associated with poor prognosis include scirrhous type, lymphatic invasion, perineural invasion, and higher proliferative index by Ki-67 [37]. Sarcomatoid intrahepatic cholangiocarcinoma is also associated with poor outcome [38]. Cholangiocarcinomas arising in the setting of intraductal papillary neoplasms have a more favorable prognosis, especially following complete resection [39].
10.1.6 Gross Findings
The liver is non-cirrhotic in 70–90 % of cases [5, 7, 40]. Intrahepatic cholangiocarcinoma is more frequently located in the right hepatic lobe (35 %) than the left lobe (22 %) [7]. The remaining tumors are centrally located (12 %) or multifocal (31 %). Most cholangiocarcinomas are solitary (Fig. 10.1), while about 30 % are multifocal (Fig. 10.2) [7, 36, 40]. Intrahepatic cholangiocarcinoma is typically firm and not encapsulated. It can be classified into three groups based on gross features, as outlined below [41–44]. However, variable combinations of these types also occur; for instance, combined mass forming and periductal infiltrating type is more common than isolated periductal infiltrating type [42].
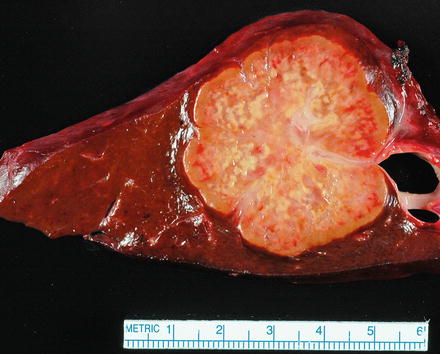
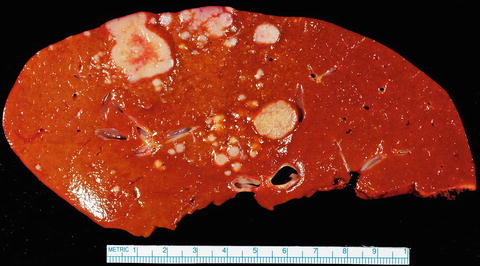

Fig. 10.1
Intrahepatic cholangiocarcinoma, single mass. The carcinoma forms a single mass with a yellow-tan color, areas of sclerosis, and irregular but well-defined borders

Fig. 10.2
Intrahepatic cholangiocarcinoma, multifocal. Multifocal tumors are less common; this example is characterized by widespread disease with numerous variably sized lesions
1.
Mass-forming type (50 % of intrahepatic cholangiocarcinomas) (Fig. 10.3): These tumors are characterized by homogeneous masses with sclerosis and irregular but well-defined and lobulated margins. Tumors have a light-tan color at the periphery and a gray-white firm center resulting from extensive fibrosis [6, 45, 46]. Hemorrhage and central necrosis are usually absent, but focal calcifications can occur. Viral hepatitis associated cholangiocarcinomas are often of the mass-forming type [47].
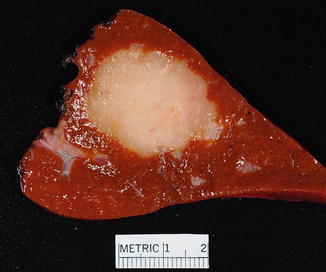

Fig. 10.3
Intrahepatic cholangiocarcinoma, single mass. A solitary cholangiocarcinoma forms a tan, fibrotic, well-circumscribed mass without evidence of periductal growth
2.
Periductal infiltrating type (9 %) (Fig. 10.4): These tumors spread along a dilated or narrowed bile duct without forming a discrete mass. This tumor type is associated with a worse prognosis.
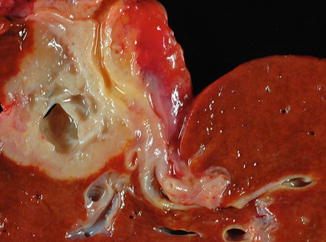

Fig. 10.4
Intrahepatic cholangiocarcinoma, periductal growth type. The periductal type of growth is characterized by tumor growth and spread along a thickened and narrowed bile duct. Various combinations can also be seen between the mass forming and periductal growth patterns
3.
Intraductal growth type (4–14 %): This type results from malignant progression of intraductal papillary neoplasms of the bile ducts and presents as polypoid or papillary tumors within the lumens of bile ducts. These tumors affect men more commonly than women and are associated with clonorchiasis and hepatolithiasis [39].
4.
Combined growth: Up to 25 % of intrahepatic cholangiocarcinomas have various combinations between the above mentioned gross growth patterns.
10.1.7 Microscopic Findings
Intrahepatic cholangiocarcinoma is typically not encapsulated (Figs. 10.5 and 10.6) and often has a dense desmoplastic response (Fig. 10.7). The vast majority of intrahepatic cholangiocarcinomas are adenocarcinomas. They have a variety of growth patterns including irregular branching tubules, infiltrating glands, solid nests, trabeculae, and sometimes micropapillary structures (Figs. 10.8, 10.9, 10.10, 10.11, 10.12, 10.13, 10.14, and 10.15). Variable growth patterns are often present within the same tumor. It is important to recognize that intrahepatic cholangiocarcinoma does not always have easily identified glandular lumens and can show a predominantly solid, trabecular, or nested growth pattern (Fig. 10.16); these patterns should be distinguished from hepatocellular carcinoma.
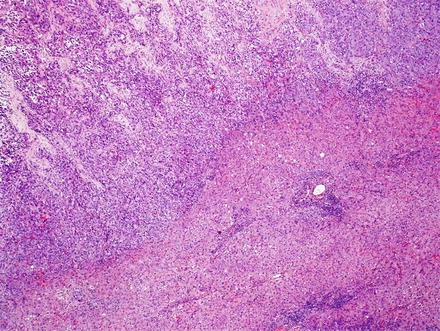
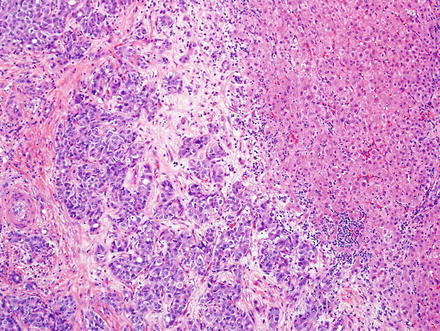
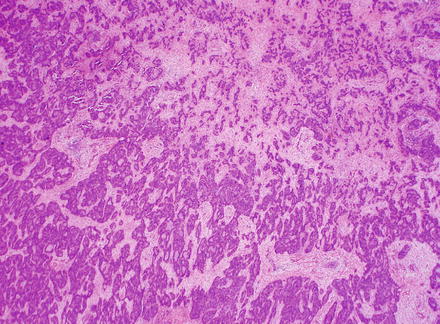
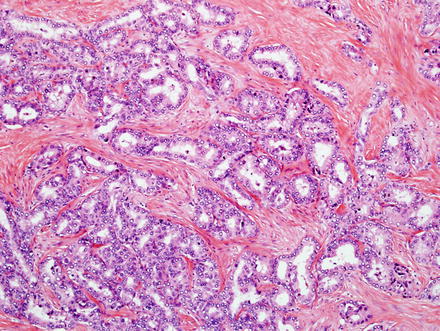
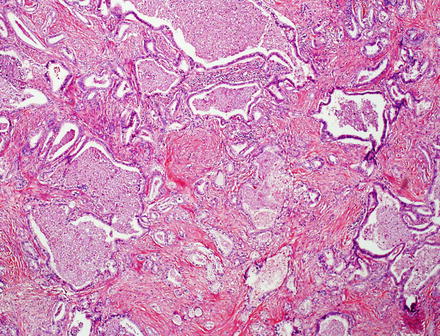
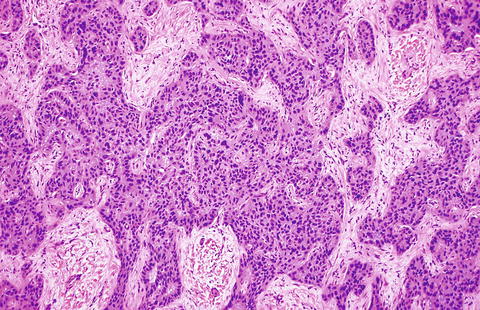
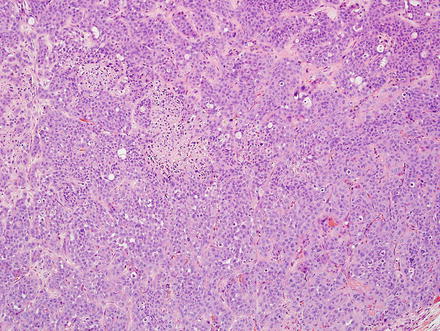
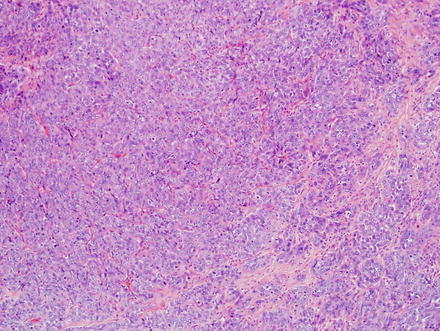
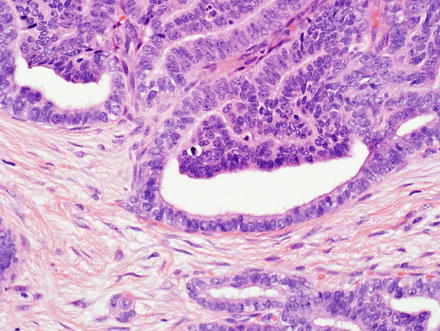
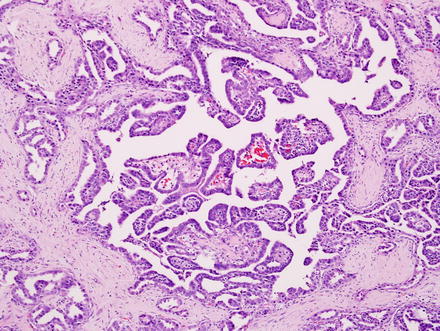
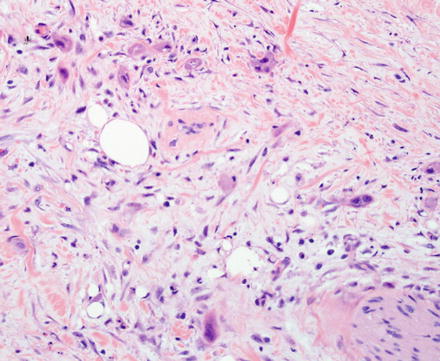
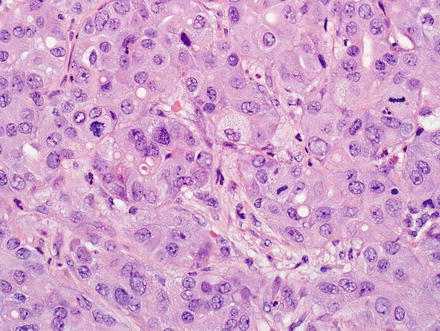

Fig. 10.5
Intrahepatic cholangiocarcinoma. Intrahepatic cholangiocarcinoma is typically unencapsulated, but usually has a sharp pushing border with the adjacent liver

Fig. 10.6
Intrahepatic cholangiocarcinoma. A higher power view of the border between cholangiocarcinoma and the adjacent non-neoplastic liver

Fig. 10.7
Intrahepatic cholangiocarcinoma. Low power examination typically shows a prominent desmoplastic stromal response

Fig. 10.8
Intrahepatic cholangiocarcinoma, glandular growth. Intrahepatic cholangiocarcinoma can form small glands

Fig. 10.9
Intrahepatic cholangiocarcinoma, glandular growth. Large glands can also be present in cholangiocarcinoma

Fig. 10.10
Intrahepatic cholangiocarcinoma, trabecular growth. This cholangiocarcinoma is growing in broad sheets and trabeculae

Fig. 10.11
Intrahepatic cholangiocarcinoma, solid growth. This cholangiocarcinoma is growing in solid sheets that mimic hepatocellular carcinoma

Fig. 10.12
Intrahepatic cholangiocarcinoma, solid growth. Another example of the solid growth pattern

Fig. 10.13
Intrahepatic cholangiocarcinoma, tubulopapillary growth. Glandular structures are seen, some of which have papillary-like projections

Fig. 10.14
Intrahepatic cholangiocarcinoma, papillary growth. This cholangiocarcinoma has a papillary growth pattern

Fig. 10.15
Intrahepatic cholangiocarcinoma. The carcinoma is infiltrating as individual cells

Fig. 10.16
Intrahepatic cholangiocarcinoma with predominant solid growth. Some intrahepatic cholangiocarcinomas can show predominantly solid, trabecular, and nested growth, with cytoplasmic eosinophilia and the absence of easily identifiable glandular lumens. Some foci can even appear to show fatty change. These growth patterns can be mistaken for hepatocellular carcinoma
Intrahepatic cholangiocarcinoma often elicits a dense desmoplastic-type fibrotic stromal response (Fig. 10.17), sometimes with hyalinization. This results from extracellular matrix production by activated myofibroblasts present in the stroma [48]. In some cases, the stroma can also show significant elastosis. Tumor necrosis and cholesterol cleft deposition (Figs. 10.18 and 10.19) can occur but are not common. Tumor cells can grow along sinusoids and spread extensively throughout the liver via the portal venous system. Perineural invasion, on the other hand, is usually seen only where the larger nerves of the liver are located (i.e. in the large portal areas close to the liver hilum). Tumor cells can also colonize and extend along the bile duct epithelium (Fig. 10.20). This growth pattern is not specific for cholangiocarcinoma and can be seen in other tumors, such as metastatic colonic adenocarcinoma.
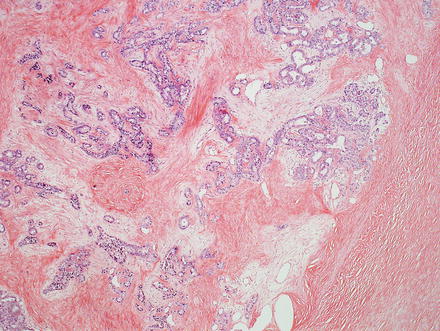
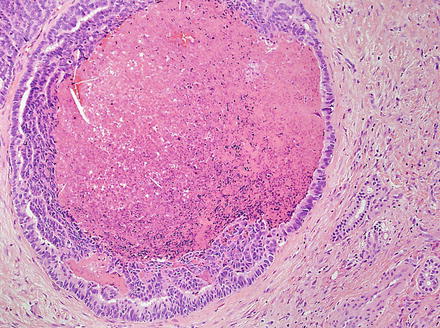
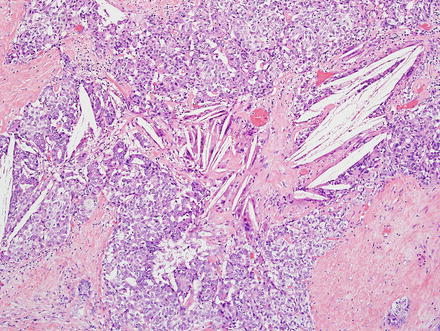
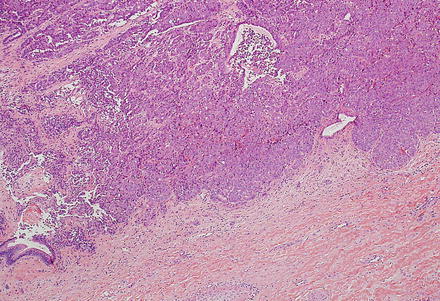

Fig. 10.17
Intrahepatic cholangiocarcinoma, stroma. Cholangiocarcinomas typically elicit prominent stromal fibrosis. This is a common feature of pancreaticobiliary adenocarcinomas in general

Fig. 10.18
Intrahepatic cholangiocarcinoma, luminal necrosis. There is necrotic debris in the lumen of this dilated gland

Fig. 10.19
Intrahepatic cholangiocarcinoma cholesterol clefts. Cholesterol cleft deposition and associated giant cell reaction is seen in this cholangiocarcinoma

Fig. 10.20
Intrahepatic cholangiocarcinoma, bile duct colonization. Cholangiocarcinoma can invade and colonize the epithelial lining of intrahepatic bile ducts. Remnants of the benign bile duct epithelium can be seen in the left lower corner
The tubules/glands of intrahepatic cholangiocarcinoma are composed of cuboidal to columnar epithelial cells; there is a rough correlation with anatomic location in that more centrally located intrahepatic cholangiocarcinomas are more likely to have columnar type epithelial cells, have well-formed glands, and produce mucin (Fig. 10.21). In contrast, cholangiocarcinomas located at the periphery are more likely to grow as irregular, anastomosing tubular structures lined by low cuboidal cells that do not produce mucin (Fig. 10.22). Peripheral tumors are also more likely to contain areas that have no apparent lumens and grow as solid trabeculae and nests. Centrally located tumors are associated with biliary dysplasia, perineural invasion, and extrahepatic recurrence, while peripherally located tumors are more commonly associated with viral hepatitis and cirrhosis [49].
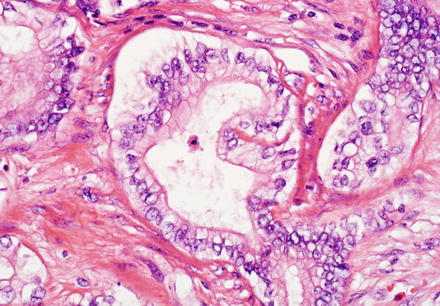
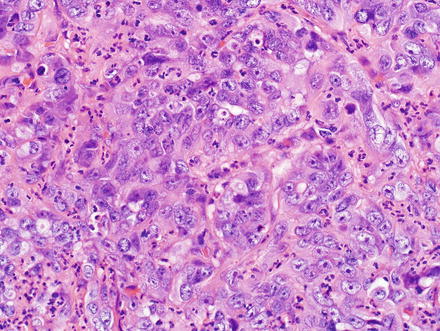

Fig. 10.21
Intrahepatic cholangiocarcinoma. The cytological features of intrahepatic cholangiocarcinoma can be variable. A well-differentiated centrally located cholangiocarcinoma features well-formed glands line by columnar neoplastic cells with a relatively large amount of apical cytoplasm

Fig. 10.22
Intrahepatic cholangiocarcinoma. This peripherally located cholangiocarcinoma shows more highly atypical cells, with higher to nuclear cytoplasmic ratio, more nuclear overlapping, and occasional ill-formed glands lined by cuboidal cells
Several morphologic subtypes of intrahepatic cholangiocarcinoma have been proposed and are described below. These subtypes are not always pure and can show a mixture of patterns. Nonetheless, the patterns are important to recognize for diagnostic, clinical, and research purposes. The predominant pattern is used to subclassify cholangiocarcinomas. Minor components (typically at least 5 %) can also be noted as being present.
10.1.7.1 Scirrhous Cholangiocarcinoma
The scirrhous subtype makes up approximately 24 % of all cases (Fig. 10.23) and is defined by cholangiocarcinomas with 70 % or more of their largest cut surface showing a scirrhous morphology (defined as more fibrous/desmoplastic component than cellular component); this subtype is associated with perineural and angiolymphatic invasion, a higher proliferative index, and lower survival. Scirrhous tumors are also associated with a mass-forming gross type [37].
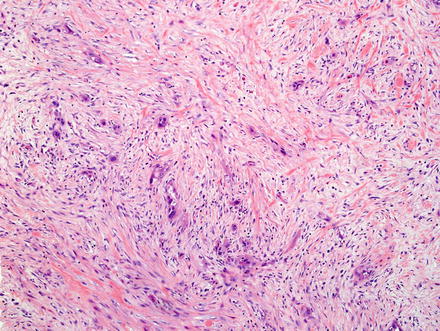

Fig. 10.23
Intrahepatic cholangiocarcinoma, scirrhous type. There is a prominent desmoplastic stromal response, which comprises the majority of the tumor, whereas the cellular component forms only a small portion of the tumor surface
10.1.7.2 Cholangiocellular Cholangiocarcinoma
Another proposed pattern/subtype of intrahepatic cholangiocarcinoma is the bile ductular or cholangiocellular pattern (Fig. 10.24); this type represents approximately 11 % of intrahepatic cholangiocarcinomas and often occurs in patients with hepatitis C [50–52]. This subtype may be arising from cholangioles and canals of Hering [53]. It also may represent a variant that derives from hepatic progenitor cells, a possibility suggested by the frequent co-expression of CK7, CK19, and NCAM [51–53]. Morphologically, the tumor consists of small tubules and arborizing, cord-like structures that have absent or slit-like lumens. There is abundant fibrous stroma, mimicking the stroma of ductular reaction in benign liver tissue. The tumor cells also resemble biliary epithelial cells lining non-neoplastic, reactive cholangioles [54, 55]. The cholangiocellular pattern of cholangiocarcinoma often shows only minimal cytological atypia and can hence be difficult to differentiate from benign bile ductular proliferations on needle biopsy [55].
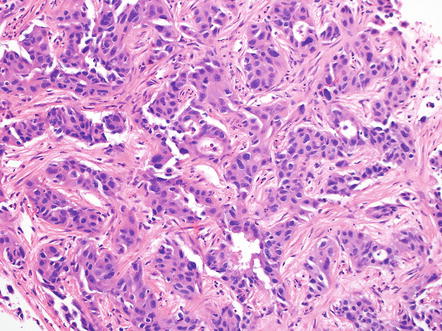

Fig. 10.24
Intrahepatic cholangiocarcinoma, cholangiocellular pattern. The cholangiocellular carcinoma has small tubular and cord-like structures with absent or slit-like lumens and abundant fibrous stroma. It can closely resemble reactive bile ductular proliferation
10.1.7.3 Ductal Plate Malformation Predominant Cholangiocarcinoma
A third proposed subtype is the ductal plate malformation predominant pattern (Fig. 10.25), a rare variant characterized by vaguely nodular carcinomatous areas composed of irregular and dilated glands, resembling ductal plate malformation, lined by a single layer of cuboidal to low columnar epithelial cells. Irregular epithelial protrusions and bulges into the dilated glands can sometimes be seen with this subtype. Most cholangiocarcinomas with the ductal plate malformation pattern occur in the setting of chronic liver disease, such as viral hepatitis or fatty liver disease [56].
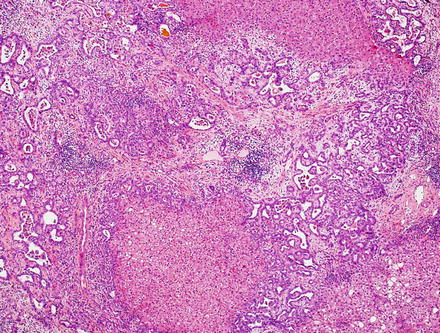

Fig. 10.25
Intrahepatic cholangiocarcinoma, duct plate malformation pattern. This variant of cholangiocarcinoma resembles a ductal plate malformation; there are vaguely nodular carcinomatous areas with desmoplastic reactions and irregularly dilated neoplastic glands. The glands have dilated lumens with irregular protrusions and bulges, sometimes containing bile, and are lined with a single layer of cuboidal or low columnar carcinoma cells
10.1.7.4 Lymphoepithelioma-Like Cholangiocarcinoma
This distinct variant of cholangiocarcinoma shows abundant intratumoral lymphocytes, both within the tumor stroma and the tumor epithelium. The tumors often have variable morphology, with some areas showing an undifferentiated component, while some other areas show well to moderately differentiated components. The poorly differentiated areas show ill-defined syncytial sheets, solid nests, or cords of undifferentiated cells with vesicular nuclei, prominent nucleoli, and indistinct cell borders. The better-differentiated areas show glandular growth pattern. Both are admixed with a lymphocyte rich stroma (Fig. 10.26). Lymphoepithelioma-like cholangiocarcinoma is strongly linked to Epstein-Barr virus (EBV); the tumor is more common in areas with endemic EBV infection, and almost all reported cases are EBV positive by in situ hybridization both in the undifferentiated and glandular components (Fig. 10.27). By immunohistochemistry, the epithelial component is positive for keratin AE1/AE3, CK7, CK19, and is negative for CEA and CK20, and the lymphocytes are predominantly T-cells. Despite limited experience due to rarity of these tumors, they appear to have a favorable prognosis [57, 58].
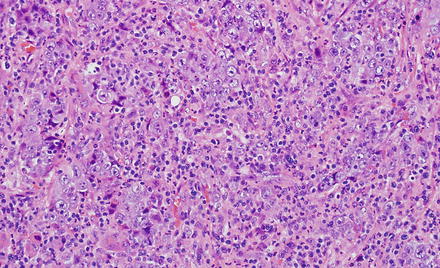
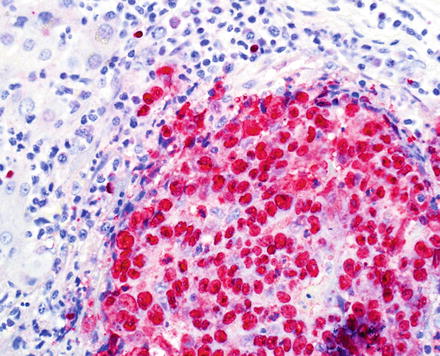

Fig. 10.26
Lymphoe-pithelioma-like cholangiocarcinoma. The tumor features an undifferentiated lymphocyte-rich carcinoma characterized by syncytial sheets of undifferentiated cells with vesicular nuclei, prominent nucleoli, and indistinct cell borders

Fig. 10.27
Lymphoe-pithelioma-like cholangiocarcinoma. In situ hybridization for EBV is positive in almost all cases. Note that the tumor cells and not the lymphocytes are positive
10.1.7.5 Rhabdoid Cholangiocarcinoma
A fifth and rare variant of cholangiocarcinoma has rhabdoid features [59, 60]. The tumors have pushing borders and feature round to polygonal cells with abundant eosinophilic cytoplasm and vesicular, eccentrically located nuclei (Fig. 10.28). Perinuclear eosinophilic inclusions are sometimes seen. The tumor cells are arranged in loosely cohesive groups separated by fibrovascular septa. More typical areas of cholangiocarcinoma growth are typically present in well-sampled tumors (Fig. 10.29). By immunohistochemistry, the neoplastic cells are positive for pan-cytokeratin, mucicarmine (faint and focal), and can have aberrant loss of INI-1 (Fig. 10.30).
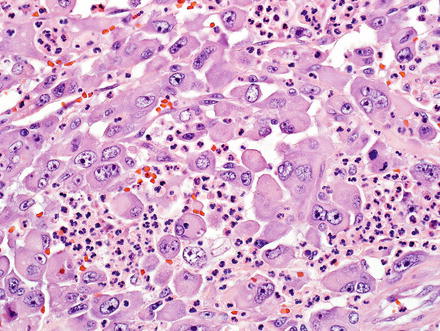
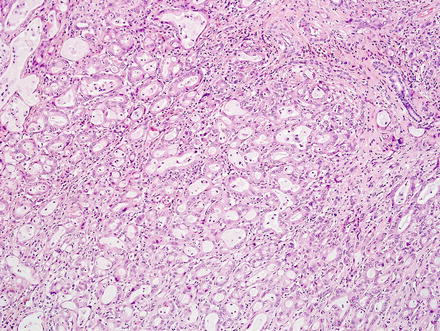
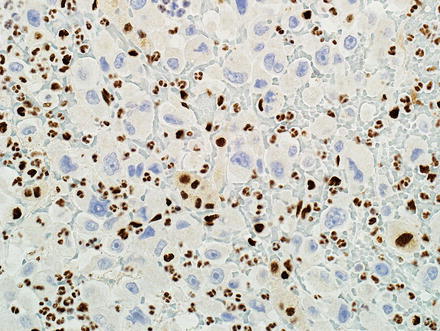

Fig. 10.28
Intrahepatic cholangiocarcinoma with rhabdoid features. The rhabdoid component consists of round to polygonal cells with voluminous eosinophilic cytoplasm, eccentrically located nuclei with prominent nucleoli, and occasional perinuclear eosinophilic inclusions

Fig. 10.29
Intrahepatic cholangiocarcinoma with rhabdoid features. Areas of a more typical cholangiocarcinoma are seen, same case in Fig. 10.28

Fig. 10.30
Intrahepatic cholangiocarcinoma with rhabdoid features. Many tumors show aberrant loss of INI-1 immunohistochemical expression in the rhabdoid component. The admixed inflammatory and stromal cells show retained expression
10.1.7.6 Additional Cholangiocarcinoma Variants
Additional rare variants include mucinous/colloid carcinoma (Fig. 10.31), which is commonly associated with intraductal papillary neoplasms of the bile ducts, hepatolithiasis and recurrent pyogenic cholangitis [7, 49, 61, 62].
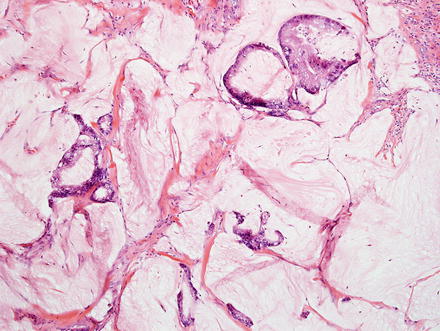

Fig. 10.31
Intrahepatic cholangiocarcinoma, mucinous. This cholangiocarcinoma consists of relatively bland tumor cells floating in large extracellular mucin lakes
Sarcomatoid cholangiocarcinomas are defined by areas of spindle cell growth (Fig. 10.32), which should make up at least 5 % of the tumor. Overall, the frequency is approximately 1–2 % of cholangiocarcinomas [63]. More typical areas with glandular morphology can be often found with sufficient tumor sampling, but only the sarcomatoid differentiation is seen in some cases [63]. The spindled cell areas grow in sheets and bundles and typically have varying amounts of pleomorphic tumor giant cells. The spindled cells should be keratin positive and negative for markers of hepatocellular differentiation. Vimentin is also positive. Tumor metastates typically retain the spindled cell morphology.
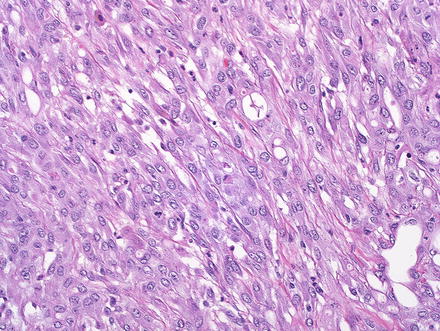

Fig. 10.32
Intrahepatic cholangiocarcinoma, sarcomatoid. This cholangiocarcinoma had large areas of spindle cell growth
Cholangiocarcinomas can sometimes produce granulocyte colony stimulating factor and have numerous intratumoral neutrophils (Fig. 10.33). Individuals with this tumor type can present with fever weight loss, high white blood cell counts, and occasionally with Sweet syndrome (neutrophilic dermatitis) [64, 65]. The diagnosis can be confirmed by immunostaining for granulocyte colony stimulating factor or by a marked drop in the leukocytosis following tumor resection. Cholangiocarcinomas that produce granulocyte colony stimulating factor often have a cholangiocellular morphology and are also more common in cirrhotic livers [66]. Some cases also have areas of sarcomatoid change [65]. Treatment effect with associated tumor necrosis can also elicit a neutrophilic response, but this is a secondary finding and does not represent the granulocyte colony stimulating factor subtype of cholangiocarcinoma.
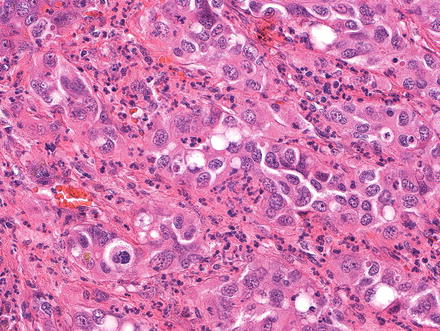

Fig. 10.33
Intrahepatic cholangiocarcinoma, granulocyte colony factor stimulating. Numerous infiltrating neutrophils are seen
Cholangiocarcinoma can have a clear cell morphology, similar to clear cell carcinomas in other organs (Fig. 10.34) [7, 67]. Rare cases of cholangiocarcinoma with signet ring cells have also been reported [68, 69]. Rare cholangiocarcinomas can also have an associated small cell carcinoma component [70].
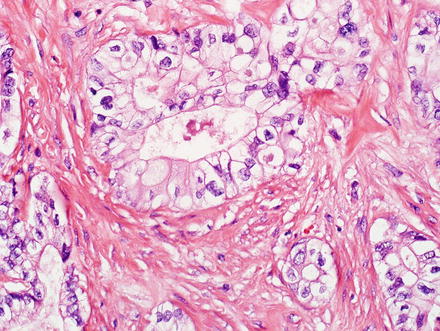

Fig. 10.34
Cholangio-carcinoma, clear cell type. This cholangiocarcinoma shows clear cells forming glands and nests
Adenosquamous cholangiocarcinoma of the bile ducts shows a component of typical glandular or trabecular cholangiocarcinoma, admixed with areas of squamous differentiation (Fig. 10.35). This pattern of cholangiocarcinoma has a poor prognosis [49, 71–74].
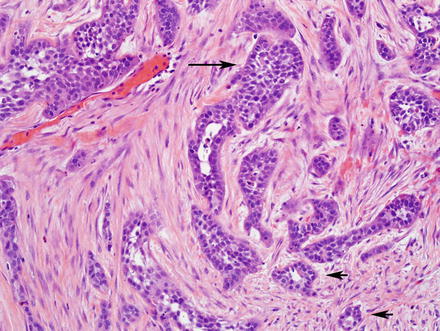

Fig. 10.35
Adenosqu-amous carcinoma. This cholangiocarcinoma has two distinct components: a squamous component (long arrow) and a glandular component (short arrows)
Squamous cell carcinomas of the bile ducts (Fig. 10.36) are also rare and associated with solitary cysts, hepatolithiasis, and fluke infection [7, 61, 75–78].
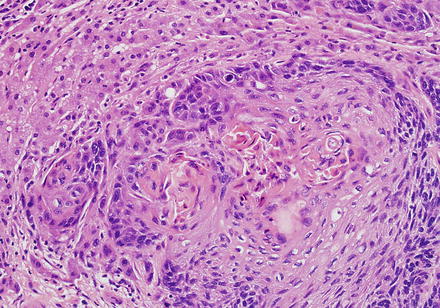

Fig. 10.36
Squamous cell carcinoma. A keratinizing squamous cell carcinoma shows a nested growth pattern
Undifferentiated carcinomas can be sarcomatoid (see above) or anaplastic and can have giant cells (Fig. 10.37). They are associated with hepatitis C and this entity is essentially a diagnosis of exclusion, after poorly differentiated hepatocellular carcinoma and metastatic disease have been excluded [6, 7, 38, 49, 79, 80]. The so-called “giant cell tumor of the liver” is composed of small undifferentiated cells, which can be focally keratin positive, and are admixed with benign reactive giant cells (Fig. 10.38). Because this tumor is commonly associated with in situ carcinoma or high grade BilIN (Fig. 10.39), it is thought to be a form of undifferentiated cholangiocarcinoma.
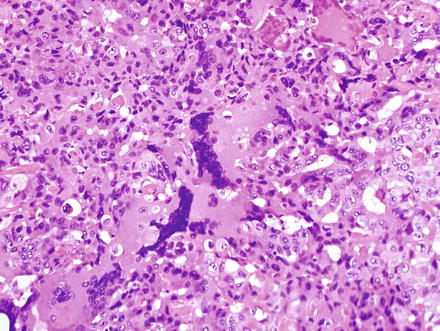
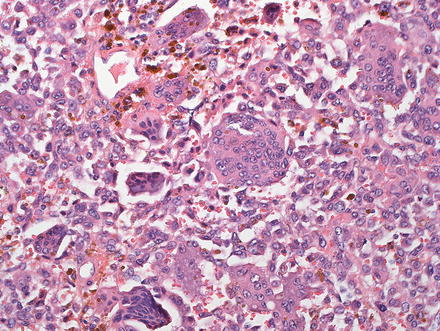
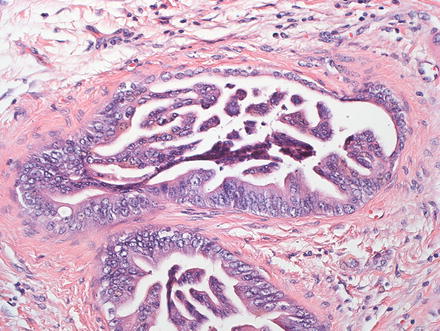

Fig. 10.37
Cholangio-carcinoma, undifferentiated. This tumor has numerous giant cells

Fig. 10.38
Giant cell tumor of the liver. This very rare tumor shows small undifferentiated tumor cells admixed with numerous benign multinucleated histiocytes. Abundant hemosiderin was also present in this case

Fig. 10.39
Giant cell tumor of the liver. Next to the mass lesion, high-grade BilIN was seen
Of note, the gross tumor morphology can correlate broadly with microscopic features [55, 81, 82]. For instance, mass-forming intrahepatic cholangiocarcinoma is associated with cholangiocellular (ductular) microscopic features, and a tendency for central tumor necrosis with higher viability in the periphery. In contrast, periductal gross morphology is associated with cholangiocarcinomas that have an infiltrative growth pattern, extending along dilated or narrowed ducts [42]. Finally, the gross intraductal growth pattern is associated with intraductal papillary biliary neoplasms; the invasive carcinomas are usually of the conventional or mucinous type.
10.1.8 Grading Intrahepatic Cholangiocarcinoma
Although universally adapted grading criteria have not been established, many pathologists utilize a four-tier grading system based on the percentage of the glandular component in the tumor; this system was adapted by the College of American Pathologists (CAP) and the American Joint Committee on Cancer (AJCC) [1]. According to this schema, adenocarcinomas are graded as follows:
1.
Well-differentiated (grade 1): greater than 95 % of the tumor is composed of glands.
2.
Moderately differentiated (grade 2): 50–95 % of the tumor is composed of glands.
3.
Poorly differentiated (grade 3): 5–49 % of the tumor is composed of glands.
4.
Undifferentiated (grade 4): less than 5 % of the tumor is composed of glands.
Tumor grade is an independent predictor of patient survival and disease recurrence [83, 84]. Therefore, it is important to include a specific grade in pathology reports in cases of cholangiocarcinoma. On the other hand, rare variants of intrahepatic cholangiocarcinoma (see microscopic findings above) cannot be graded according to this scheme and are usually not assigned a specific grade. Although some have suggested assigning specific grades to signet ring adenocarcinoma (grade 3) and small cell carcinoma (grade 4) [83], reporting the specific variant is more important than assigning a tentative grade.
10.1.9 Staging
Several pathologic features are independent prognostic factors in cholangiocarcinoma, including angiolymphatic invasion, intrahepatic metastasis, lymph node metastasis, and periductal macroscopic tumor type [37, 40, 44]. These features are incorporated into the American Joint Committee on Cancer (AJCC) TNM tumor staging scheme (7th edition) (Table 10.2) [1]. Other predictors of outcome include tumor size, positive margins, the presence of satellite nodules, and the degree of tumor necrosis [85].
Table 10.2
Staging of intrahepatic cholangiocarcinoma according the American Joint Committee on Cancer
Primary tumor (T) | |
TX | Cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ (intraductal tumor) |
T1 | Solitary tumor without vascular invasion |
T2a | Solitary tumor with vascular invasion |
T2b | Multiple tumors, with or without vascular invasion |
T3 | Tumor perforating the visceral peritoneum or involving the local extrahepatic structures by direct invasion |
T4 | Tumor with periductal invasion |
Regional lymph nodes (N) | |
NX | Cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
10.1.10 Immunohistochemical Features
Intrahepatic cholangiocarcinoma has an immunoprofile that is similar to that of other pancreaticobiliary and upper gastrointestinal adenocarcinomas. There are currently no positive affirmative stains to identify cholangiocarcinoma. Instead, the diagnosis is based on exclusion of other tumors using morphology, immunostains, imaging studies, and clinical findings. Once other carcinomas have been excluded, a diagnosis of cholangiocarcinoma can be made.
Intrahepatic cholangiocarcinoma typically shows cytoplasmic staining with polyclonal CEA (pCEA). CK19 is also positive in 70–80 % of cases, while MOC31 is positive in approximately 90 % of cases [86]. Intrahepatic cholangiocarcinoma is virtually always positive for CK7, but varies in CK20 expression. Various studies have shown that the immunoprofile of cholangiocarcinoma can depend on its location. For instance, approximately 50 % of peripheral cholangiocarcinomas are CK20 negative, while central and extrahepatic cholangiocarcinomas tend to be CK20 positive [87]. Likewise, peripheral cholangiocarcinomas, particularly those with a “bile ductular” pattern, tend to express CD56 [88]. Furthermore, focal positive staining with HepPar1, though rare, is more commonly seen in peripherally located tumors; this immunoprofile should not be mistaken for a combined hepatocellular carcinoma/cholangiocarcinoma. Immunostains for S100P can be positive in cholangiocarcinoma and this can help in differentiating cholangiocarcinoma from florid bile ductular proliferations, which are usually negative for S100P.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








