Fig. 16.1
Illustration depicting air-dried cervical mucus studied with light microscopy (× 40). a Es mucus: a parallel arrangement of crystals is close together but not joined, with or without short branches protruding from them. b El mucus: a typical ferning morphology. It has a structure composed of straight or curved central axis and with branches protruding from its axis at a 90° angle. These branches can also act as an axis for new branches, again at a 90° angle. c G mucus: a high free-crystal content with no predetermined form
Ultrastructurally, the cervical mucus can be seen as a complex biphasic fluid with high-viscosity and low-viscosity components. It is a hydrogel composed of a low-molecular-weight component (cervical plasma) and a high-molecular-weight component (gel phase). The cervical plasma consists mainly of trace elements (zinc, copper, iron, manganese, selenium, sodium, and chloride ions), organic components of low molecular weight such as glucose and amino acids, and soluble proteins, such as albumin and globulins [12, 55, 56]. The gel phase consists of a glycoprotein network called mucin, presenting glycosylation variations according to the menstrual cycle, contributing to the changes in its physical properties. This extremely large macromolecule (about 10,000 KDa) is rich in carbohydrate content and is responsible for the high mucus viscosity [57]. The mucin macromolecules are thread-like and appear in long parallel bundles maintained by a peptide of 30 kDa. This peptide connects mucin molecules through disulphide bridges (S–S), thus forming mucin micelles of 100–1000 glycoprotein chains [55]. This system assembly, which varies both in diameter and arrangement, is called “micelle.” Collectively, mucin molecules form a complex of interconnected micelles, which comprise a lattice whose interstices are capable of supporting the low viscosity phase, which is predominantly water. Protein content is low in the intermicellar spaces of Es mucus. The very low viscosity of Es intermicellar fluid allows very rapid sperm migration [12, 58]. In type G mucus, no micelle formation occurs, but the long macromolecules form a large, three-dimensional, irregular, dense network that does not allow spermatozoa to penetrate (Fig. 16.2) [59]. These channels or spaces vary in size according to the type of mucus: 2–5 μm wide (Es type mucus), 1–2 μm wide (El type mucus), and 0.3–1 μm wide (type G mucus). Therefore, intermicellar spaces play a key role in sperm migration [12] .
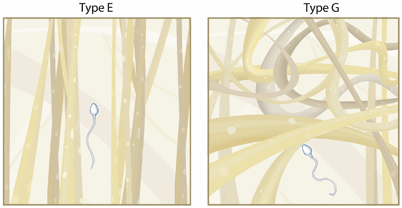

Fig. 16.2
Schematic representation of the gel structure of types E and G cervical mucus. In type E, mucin macromolecules lie together in long parallel bundles (micelles), which spaces in between are filled with cervical plasma. Spermatozoa can easily swim through these spaces. In type G, no micelle formation occurs, and long macromolecules form a large, three-dimensional, irregular, dense network. Spermatozoa are not allowed to penetrate
Abnormalities of cervical mucus can result in infertility. For instance, chronic cervicitis is associated with alterations of cervical mucus. In this case, a different mucus pattern appears, defined as type Q by Odeblad, in which the mucus composition varies depending on the type, degree, and duration of the inflammatory process. The crypts releasing this type of secretion have limited response to hormonal stimulation [51, 60]. In acute inflammatory conditions, the crypts can also produce a serous type of secretion of low viscosity but with high leukocyte content, classified as type V, which is unable to maintain sperm vitality. Therefore, common infections of the cervix such as those caused by sexually transmitted microorganisms ( Chlamydia trachomatis, Neisseria Gonorrhea, Trichomonas vaginalis, Mycoplasma hominis, and Ureaplasma urealyticum) may result in cervical hostility [12].
Women with cystic fibrosis (CF) are also unable to produce the watery and stretchy mucus needed for optimal sperm penetrability. CF is caused by a mutation in the gene for the protein CF transmembrane conductance regulator (CFTR). This protein functions as a channel which transports negatively charged particles (chloride ions) inside and outside the cells. The transport of chloride ions helps to control the movement of water in tissues, which is necessary for the production of thin, freely flowing mucus. When the CFTR protein does not work properly, chloride (Cl−) is trapped inside the cells. Because chloride is negatively charged, it creates a difference in the electrical potential inside and outside the cell causing sodium to cross into the cell. As a result, water movement from inside to outside cellular compartments is decreased, leading the mucus to be more viscous and less watery thus harming the sperm transport . Along with a loss of Cl– conductance, mutations of CFTR protein also impede bicarbonate (HCO3 −) transport. A HCO3− rich alkaline pH environment is crucial for optimal sperm motility and capacitation. During the process of releasing highly condensed mucins from intracellular granules, calcium (Ca2+) and hydrogen (H+) cations must be removed to enable the mucins to expand by as much as 1000 times, forming extracellular mucus–gel networks. It is suggested that HCO3 − is essential to normal mucin expansion because it forms complexes with these cations. Due to defective HCO3 − secretion in CF, mucins tend to remain aggregated, poorly solubilized, and less transportable. It is tempting to consider that some cases of reduced fertility in females might be associated with putative mild mutations in this gene with consequent abnormal cervix-uterine mucus release due to inadequate HCO3 − secretion [14, 61, 62]. An example of this condition is the report of two infertile sisters with significantly abnormal cervical mucus who were found to be compound heterozygote carriers of the CF ΔF508 and R117H/7T mutations [13].
Many exogenous factors can render the cervical mucus hostile to sperm and, therefore, be implicated in the pathophysiology of unexplained infertility [12]. Clomiphene citrate (CC) , frequently used to stimulate follicle growth and ovulation as a first line therapy in couples with unexplained infertility, can interfere with the cervical mucus. Clomiphene is structurally similar to estrogen, which allows CC to bind to estrogen receptors (ER) throughout the reproductive system. In contrast to estrogen, CC binds to nuclear ER for extended periods of time, that is, weeks rather than hours, which ultimately depletes ERs by interfering with the normal process of ER replenishment. Acting at the hypothalamic level, CC is effective in ovulation induction by inhibiting negative feedback of estrogen on gonadotropin release, leading to up-regulation of the hypothalamic–pituitary–adrenal axis that, in turn, serve to drive ovarian follicular activity. At the same time, CC exerts undesirable and unavoidable adverse antiestrogenic effects in the periphery (endocervix and endometrium). Several studies have described that CC has adverse effects on the quality and quantity of cervical mucus based on cervical mucus score, the value of which is debatable. Despite that, available evidence and accumulated clinical experience support the notion that any adverse antiestrogenic effect presents a significant obstacle for the largest majority of women treated with ovulation induction drugs [16–18, 63, 64]. Another drug that deserves attention is propranolol, which accumulates extensively in the cervical mucus after oral administration; its concentration is fourfold higher in the mucus compared with in the blood. Despite not affecting mucus production, propranolol accumulation may impair sperm motility by its direct effect on sperm membrane ion transport and energy production [19, 65–67]. Nicotine and its metabolite cotinine are secreted into the cervical mucus, and can be found in the mucus even of passive smokers [68, 69]. A retrospective study evaluating smoking histories of 901 women with infertility due to different etiologies and 1264 pregnant women admitted for delivery suggested that smoking is a risk factor for cervical factor infertility (relative risk = 1.7; 95 % confidence interval of 1.0–2.7); however, its mechanism of action is unclear [70]. It has been suggested that nicotine could have toxic effects on spermatozoa, but in vitro studies have noted that the harmful effects of nicotine and cotinine to sperm occurs in extremely high concentrations, not seen in the seminal plasma or cervical mucus of smokers [20, 71].
Other Components of Cervical Mucus
The cervical mucus contains not only mucin but also other proteins such as albumin and globulin. The concentration of different proteins in the mucus varies during the menstrual cycle, being lowest at ovulation . Morales et al. (1993), studying the mucus’ concentration of protein and its ability to sustain sperm migration, found that periovulatory mucus exhibited low protein concentrations as revealed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Most of the soluble proteins found in the cervical mucus had their lowest concentration around the periovulatory period, when the mucus is most receptive to sperm penetration [72]. The authors of the aforementioned study concluded that there is a statistically significant inverse relationship between protein concentration in the mucus and its ability to sustain sperm migration.
The mucus also provides local immunity through a unique interaction of immunoglobulins (mainly IgA), cytokines, and reproductive hormones (estrogen and proegesterone). Also, the mucus is a rich source of antimicrobial proteins and peptides, including secretory leucocyte protease inhibitor (SLPI), lysozyme, calprotectin, lactoferrin, human neutophil peptides 1–3, and epithelial beta-defensin [12, 73]. Increased levels of cervical mucus IgA and IgG have been reported in 23 women with genital infections caused by N. gonorrhea, T. vaginalis, genital herpes, and nonspecific cervicitis in comparison with a control group of 23 uninfected women ( p< 0.001), thus indicating increased local immune response [74]. Furthermore, it has been suggested that leukocytes, mainly neutrophils, play a role in both the cervical cellular defense line and the “selective” mechanisms of sperm transport through the cervix (phagocytosis of abnormal spermatozoa) [75].
Prostagladins and trace elements also have hormone-dependent cyclical variation in the cervical mucus during the menstrual cycle. Prostaglandins found in the cervical mucus are PGE1, PGE2, PGD2, PGF1α, and PGF2α, and their contents increase in the preovulatory period. However, their biological importance remains unclear [76]. Ryantová et al. (2008), evaluating PGE2 levels in ovulatory cervical mucus of 120 women with unexplained miscarriages, found that PGE2 levels were 6 ×, 13 ×, and 21 × higher in patients with one, two, and three or more miscarriages compared with controls ( p< 0.033), respectively [77]. Iron and copper levels show marked fall from preovulatory to ovulatory phases. Interestingly, their levels in the cervical mucus were elevated both in patients with primary or secondary infertility couples compared with fertile counterparts. The real influence of these elements in fertility remains unclear, but a spermatotoxic effect of copper has been described [55, 78–80].
Table 16.1 summarizes the conditions that may affect fertility at the vaginal and cervical levels.
Table 16.1
Factors affecting fertility at the vaginal and cervical levels
Vagina | Increase in the vaginal pH: alteration in the vaginal flora, leading to an increased susceptibility to infectious processes (phagocytosis of sperm, proinflammatory cytokines) |
Sperm deficiency in neutralizing the vaginal pH: hypospermia and deficient-seminal vesicle secretion | |
Deficiency in sperm liquefaction (e.g., abnormal prostatic secretions) | |
Use of lubricants toxic to sperm | |
Cervix | Previous surgery (e.g., cauterization, cone biopsy, and curettage) |
Infections | |
Müllerian abnormalities | |
Exogenous: intrauterus diethylstilbestrol (DES) | |
Cervical mucus | Hormonal: abnormal estrogen levels and premature progesterone rise |
Inflammatory: chronic cervicitis/acute inflammatory conditions | |
Genetics: cystic fibrosis | |
Exogenous: clomiphene citrate, propranolol, nicotine | |
Trace elements: excess levels of copper, iron, and selenium | |
Male-related: asthenozoospermia and abnormal morphology | |
Immunological: antisperm antibodies in the female serum and semen |
Sperm Transport Through the Cervical Mucus
Sperm movement inside the cervical mucus occurs primarily through the interstitial spaces in the mucin micelles. Sperm progression depends mainly on the size of these spaces [81]. The spaces between these large glycoproteins reach their maximum at the midcycle estrogen peak, when there is an increase in mucus production and in its water content [53].
Besides hormonal factors, uterine contractions can also alter the spaces between these macromolecules by mechanical pressure. Furthermore, these mechanical forces contribute to the orientation of the mucin filaments. It is suggested that the outward flow of the cervical mucus establishes a linear alignment of parallel strands, creating aqueous channels between the filaments that direct sperm upward [2, 7, 58]. Given this longitudinal orientation, with mucus outflow originating in the crypts of the cervical epithelium, it has been postulated that spermatozoa are constrained to swim in the direction of least resistance, that is, along the tracts of mucus outflow in the direction of the cervical crypts [82, 83]. This theory is in agreement with the notion that spermatozoa entering the cervix are directed toward the cervical crypts, which are the sites of mucus secretion that serve as possible sperm storage reservoir. The number of spermatozoa within the cervical mucus is relatively constant for the first 24 h after coitus. Spermatozoa may retain their fertilizing capacity in the human cervical mucus for up to 48 h and their motility for as long as 120 h after ejaculation. However, the number of motile sperm within the mucus is markedly decreased after 48 h [84–86] . From their temporary storage location within the cervical crypts, sperm can be released gradually over time, thus enhancing the probability of fertilization . As the size of the interstices is usually smaller than the size of sperm heads, spermatozoa must actively push their way through the mucus. Therefore, one cause of infertility, presumably, is the reduced sperm progressive motility that prevents sperm movement through the mucus [2, 7] .
It is generally believed that another potentially important feature of human cervical mucus is its ability to restrict migration of abnormal spermatozoa, thus acting as a “filter” that eliminates deficient sperm [21, 87, 88]. It has been shown that abnormal sperm have poorer hydrodynamic profile compared with morphologically normal motile sperm [7, 21, 87, 88]. Moreover, sperm movement is probably influenced by the interaction between the mucus and the surface properties of the sperm head; for example, sperm antibodies on the sperm head inhibit sperm movement through the mucus [89].
Like the vagina, the cervix can assemble immune responses. Studies have shown that vaginal insemination stimulates the migration of leukocytes, particularly neutrophils and macrophages, into the cervix as well as into the vagina [90, 91]. This leukocytic invasion protects against microbes that are often seen in the semen, but it does not present a barrier to normal sperm under physiological conditions [88]. On the other hand, it has been demonstrated that neutrophils bind to and ingest human sperm if the female serum contain both serological complement and complement-fixing antisperm antibodies (ASA) [92]. This process occurs when the female becomes immunized against sperm antigens. As already mentioned, immunoglobulins, mainly IgG and IgA, have been detected in human cervical mucus. Secretory IgA is produced locally by plasma cells in subepithelial connective tissue. Although immunoglobulins provide protection from microorganisms, immunological infertility can occur when antibodies present in the cervical mucus recognize sperm-bound antigens [93]. Since complement proteins are present in the cervical mucus, antibody-mediated sperm destruction as well as leukocytic sperm capture may occur [94] . Despite the fact that not all ASA are complement-activated, they can still interfere with sperm progression by attaching the sperm head and avoiding spermatozoa to enter the microarchitecture of the cervical mucus network [93, 95]. Furthermore, the presence of ASA in the male can also result in infertility since such antibodies have been shown to affect sperm motility and function [22, 23, 36] .
Conclusions
A substantial diminution in sperm number occurs as they transverse the cervix. From an average of 200 to 300 million sperm deposited in the vagina, only a few hundred achieve proximity to the oocyte. Given this expected high spermatozoa loss, slight modifications in the vaginal pH and cervical mucus may rapidly transform these compounds into a “hostile” environment that may prevent natural conception and be a cause of infertility. Among the several conditions that may be involved in the pathophysiology of unexplained infertility at the vaginal and cervical levels, physicians should pay particular attention to (1) inadequate buffering capacity of acid vaginal pH, (2) alterations in cervical anatomy caused by surgeries, birth defects, and infections, and (3) alterations in the cervical mucus caused by hormonal dysfunctions, inflammatory disorders, CF, exogenous and immunological factors.
Acknowledgment
The authors are grateful to Mrs. Fabiola C. Bento for language revision.
References
1.
Kelly KG. Tests on vaginal discharge. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990. p. 833–5.







