Fig. 11.1
Normal duodenal villi after (a) air insufflation and (b) water immersion
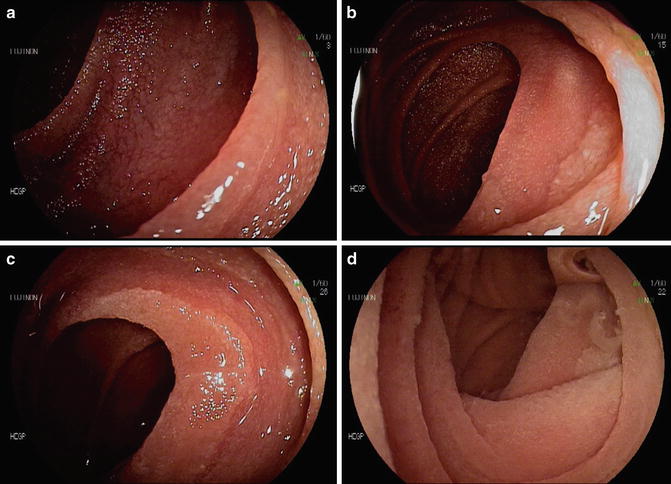
Fig. 11.2
Endoscopic features suggestive of celiac disease. (a) Mucosal fissures, grooves, and scalloping of the duodenal mucosa. Focal villous atrophy with (b, c) air insufflation and (d) after water immersion
Reduction or loss of duodenal folds, with a sensitivity of 47–88 % and a specificity of 83–97 % [7, 8].
Scalloping of the mucosa, described as a notched and nodular appearance of the duodenal folds, with a sensitivity varying from 6 to 44 % [8, 9] and a specificity of 94–100 % [8, 10].
Mosaic pattern or cobblestone appearance of the duodenal surface, with a sensitivity of 12 % and a specificity of 100 % for CD [8].
Nodularity, also described in the duodenal bulb [11], has a sensitivity of 6 % and a specificity of 95 % for the diagnosis of CD [8].
The presence of any endoscopic marker of CD has a sensitivity of 37–94 % [14, 15] and a specificity of 92–100 % [15, 16]. The most reliable endoscopic marker in terms of sensitivity appears to be the loss of the duodenal folds, however, with very heterogeneous values among published studies [17]. The specificity of these endoscopic markers of CD is good, with numbers ranging from 92 to 100 % [17]. Among differential diagnoses, nonceliac causes of villous atrophy, such as Whipple’s disease, enteropathy associated with primary hypogammaglobulinemia, autoimmune enteropathy, drug-induced toxic enteropathies (angiotensin II receptor blokers, mycophenolate mofetil), and tropical sprue should be considered [4, 18].
These specific endoscopic markers left aside, the judgment of the endoscopist on possible villous atrophy seems to be quite reliable. Although conducted without high-resolution videoendoscopes in most studies, white light endoscopic examination alone could predict the diagnosis of villous atrophy in more than 50 % of cases [10]. A study including 87 patients in an expert endoscopy center even reported sensitivity, specificity, and positive and negative predictive values of 94 %, 100 %, 100 %, and 96 %, respectively [12]. The ability of white light endoscopic examination alone to predict villous atrophy is, however, uncertain, since villous atrophy may be patchy, and early stages of villous atrophy are not easily identified by endoscopy [18, 19]. Hence, mucosal biopsies should be performed even in an endoscopically normal duodenal mucosa.
Advanced Endoscopic Imaging Techniques
Numerous techniques have been assessed to improve the visualization of the mucosal pattern in the duodenum. First and foremost, Cammarota et al. have demonstrated the interest of the “immersion technique”; i.e., the observation of duodenal mucosa after air exsufflation and instillation of 90–150 mL of water in the duodenal lumen. As presented in Figs. 11.1 and 11.2, and confirmed by several studies from this group, the water immersion technique could improve the sensitivity of upper endoscopy for the diagnosis of villous atrophy to more than 90 % [20]. However, these promising results have not been confirmed by other teams.
Chromoendoscopy appears to enhance the borders of flat lesions in the colon, stomach, and duodenum, but there is little data in the literature to suggest a benefit of dye spraying in the duodenum to increase the detection of villous atrophy. Methylene blue chromoendoscopy, even in expert hands, did not bring any improvement in the diagnosis of villous atrophy [12]. Indigo carmine in combination with magnification endoscopy showed greater than 90 % sensitivity for villous atrophy, including partial villous atrophy [13]. A second work confirmed the interest of indigo carmine dye spraying, with or without magnification endoscopy, to improve the detection of villous atrophy, especially in the duodenal bulb [21].
Magnification endoscopy may improve the sensitivity of endoscopy for the diagnosis of villous atrophy, with numbers ranging from 90 to 100 %, either alone [22–24], associated with acetic acid [14] or indigo carmine dye spraying [13]. Cammarota et al. even reported a 100 % sensibility of magnification endoscopy coupled with the water immersion technique [23].
Optical coherence tomography (OCT), an imaging technique similar to the B mode ultrasonography, used by ophthalmologists to assess retinal disorders, has recently been applied to digestive endoscopy. Masci et al. have reported in two studies an acceptable concordance between villous atrophy diagnosed by OCT and pathological examination of duodenal biopsies, resulting in a sensitivity of 82 % and a specificity of 100 % [25, 26].
Endocytoscopy is a novel diagnostic technique allowing for in vivo real-time visualization of the mucosa under 450× magnification. This noninvasive technique has been shown to be useful in in vivo and real-time and can adequately characterize the villous architecture of the duodenal mucosa in patients with celiac disease. Moreover, endocytoscopy accurately identifies mucosal histopathology of advanced CD [27, 28].
The potential contribution of confocal endomicroscopy to the diagnosis of celiac disease has been evaluated of course, with the promising capability of assessing both the degree of villous atrophy and the density of intraepithelial lymphocytes. Three studies have been published to date, all using a device from Pentax® which is currently unavailable [29–31]. Despite an 80–100 % overall specificity, sensitivity values for villous atrophy, crypt hyperplasia, and intraepithelial lymphocyte infiltration were 70–74 %, 52 %, and 81 %, respectively.
In conclusion, advanced endoscopic imaging techniques have not changed much over time in the endoscopic evaluation of a patient with suspected CD. The evidence thus far for clinical practice suggests that high-definition white light upper videoendoscopy and careful examination of the mucosa for patchy lesions are the most effective methods and remain the standard protocol for diagnosing CD. Recent studies suggest that water immersion and/or indigo carmine dye spraying preceding mucosal biopsies may also be helpful.
Intestinal Biopsy Technique
Intestinal mucosal biopsies remain the cornerstone of the diagnosis of CD. They should be repeated after 6–12 months on a gluten-free diet, in order to assess the healing of duodenal mucosa and the new growth of duodenal villi. Complete mucosal healing is variable in adults, and usually requires 2–3 years [32]. However, complete mucosal healing of the duodenum has been associated with a good prognosis, because of a lower rate of T cell lymphoma [33]. Hence, the American College of Gastroenterology clinical guidelines recommend that the first assessment of villous architecture recovery of duodenal histology should wait until 2 years on a gluten-free diet (even in case of symptom regression and normalization of antibody levels), or 6–12 months in case of nonresponsive CD [4].
The current guidelines recommend that the endoscopist should obtain at least four biopsies from the second portion of the duodenum and one or two biopsies in the duodenal bulb [4]. Indeed, villous atrophy, along with other histological abnormalities, can be patchy in the small intestine [34], and the number of 4 is a cutoff above which the sensitivity of biopsies rises significantly [35]. About 10 % of patients with CD have a villous atrophy restricted to the duodenal bulb [36]: hence, one or two biopsies, ideally in the 9 and 12 o’clock positions, should be added to the four biopsies from the second portion of the duodenum [4]. However, gastroenterologists should be aware of potential pitfalls in the interpretation of these duodenal bulb biopsies, due to peptic duodenitis or the epithelial changes in the immediate vicinity of Brünner’s glands.
Other Endoscopic Findings in Celiac Disease Patients
Villous atrophy left aside, gastroesophageal reflux disease is the most prevalent endoscopic finding in celiac patients, and interestingly, dyspeptic symptoms typically regress under a gluten-free diet. Celiac disease is associated with a decrease in the basal pressure of the lower esophageal sphincter and peptic esophagitis is twice as frequent in CD patients as in nonceliac dyspeptic controls [38]. Along with this finding, the prevalence of Barrett’s esophagus may be twice as high in CD patients as in controls [39]. Nonerosive gastric mucosal lesions, typically varioliform, have also been reported to be associated with CD. Histologically, they present as lymphocytic gastritis, of which the significance is still under debate [18].
Ulcers, strictures, or protruding lesions can be observed in any part of the small bowel in CD patients. These lesions are highly suspicious of a malignant T cell proliferation or, more seldom, of an adenocarcinoma, and should be biopsied. Strong consideration should be given for sending the patient to an expert center.
Intraepithelial lymphocytic infiltrate of the terminal ileum has been reported in association with CD, and this finding should lead the endoscopist to perform duodenal biopsies to rule out villous atrophy [40]. Finally, CD is more frequently associated with other digestive conditions, such as microscopic colitis (either lymphocytic or collagenous colitis) or inflammatory bowel disease. These conditions should be searched for in case of persistent diarrhea or abdominal pain on a strict gluten-free diet [18].
Small Bowel Capsule Endoscopy in Celiac Disease
Small bowel capsule endoscopy (CE) is a promising technique in the field of CD because of its ability to image the entire small bowel mucosa with high-quality pictures. Of note, the presence of fluid enhances the visualization of intestinal villi. Furthermore, it is less invasive than upper endoscopy, and thus more acceptable for patients. The main limitation of CE in CD is the risk of capsule retention proximal to a stricture, but this is relatively rare in this setting. However, radiological imaging of the small bowel using magnetic resonance (MR) or computed tomography (CT) enterography or the patency capsule should always precede CE in the presence of abdominal pain compatible with obstruction and/or a small bowel stricture.
The sensitivity of CE for the diagnosis of CD ranges from 77 to 92 %, with a specificity of 91–100 % [41, 42]. These diagnostic performances may be even better than those of optical endoscopy. However, histological assessment of the duodenal mucosa remains mandatory to establish the diagnosis of CD, and CE remains restricted to patients with a high clinical and biological suspicion of CD and who either refuse upper endoscopy, or have normal or indefinite biopsies for CD [41]. The endoscopic markers of CD are the same as in optical endoscopy: reduced duodenal folds, scalloping of folds, mucosal fissures, crevices or grooves, mosaic pattern, and visible submucosal vessels. These signs should draw the attention of the gastroenterologist interpreting a small bowel capsule study and typical features can be seen (Fig. 11.3 and Video 11.2).
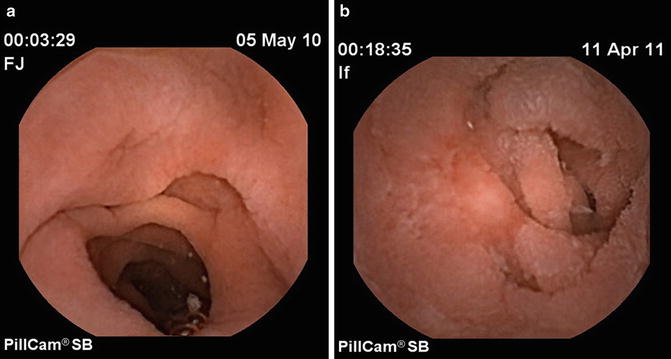

Fig. 11.3
Endoscopic features of celiac disease on capsule endoscopy. (a) Villous atrophy and scalloping, and (b) jejunal ulcer in the setting of ulcerative jejunitis
The biggest impact of CE in CD is the diagnostic workup of nonresponsive or complicated CD. In nonresponsive CD, after 6–12 months of a well-conducted gluten-free diet, the patient should undergo further diagnostic investigations, searching for causes of refractory celiac disease. Once radiological imaging of the small bowel has excluded a digestive stricture, CE should be considered to search for small bowel ulcers, especially located beyond the distal duodenum. The results of the capsule study can aid the endoscopist in choosing between standard esogastroduodenoscopy, push enteroscopy, or deep enteroscopy [43, 44]. It should be noted that there are currently no national or international guidelines to support this management, given the relatively high prevalence of small bowel mucosal ulcers (generally attributable to nonsteroidal anti-inflammatory drug [NSAID] intake) in CE procedures performed in uncomplicated CD patients [45]. CE could also play a role in the annual follow-up of patients with refractory celiac disease, by searching for jejunal or ileal mucosal ulcers suggesting ulcerative jejunitis or malignant transformation of CD.
Role of Enteroscopy in Celiac Disease
Push enteroscopy, balloon-assisted enteroscopy, and/or spiral enteroscopy have very few indications in CD, especially since the development of small bowel capsule endoscopy. It is a time-consuming and invasive procedure, carrying a risk of perforation in patients with diseased small bowel. Rarely, does enteroscopy add much in the evaluation of CD. Since mucosal lesions are known to be patchy in CD, some physicians have advocated for enteroscopy in order to obtain jejunal mucosal samples, particularly when duodenal biopsies are normal. However, several studies have shown that the diagnostic yield of jejunal biopsies in this setting is insignificant. Cellier et al. demonstrated that enteroscopy did not change the management of responsive CD patients [46]. In another study including more than 140 patients, Thijs et al. found a 2 % rate of pathological jejunal biopsies when duodenal biopsies were normal [47]. Meijer et al. reported a 6 % clinically significant discrepancy between duodenal and jejunal biopsies in more than 100 celiac patients [48]. Given the 96 % sensitivity of the duodenal biopsies (including duodenal bulb biopsies) for the diagnosis of CD, there is little data to support routine upper enteroscopy in the diagnosis of CD for patients with positive serology and negative duodenal biopsies [4].
In contrast, for nonresponsive or complicated CD, enteroscopy remains a useful diagnostic tool. Refractory celiac disease is defined by the persistence of villous atrophy after at least 6 months on a strict gluten-free diet, in the absence of another cause of villous atrophy [1]. Type I disease, in which the phenotype of intraepithelial lymphocytes is normal, should be distinguished from type II, in which clonal expansion of abnormal intraepithelial lymphocytes is observed. This latter form is actually a low-grade T-cell lymphoma and carries a more ominous prognosis. In this setting, enteroscopy allows for the histological follow-up of the duodenal and jejunal mucosa through systematic and targeted mucosal biopsies [43, 49]. Unlike uncomplicated CD, mucosal abnormalities with ulcerative jejunitis can be limited to the jejunum, without any ulcers seen in the duodenum (Fig. 11.3, Video 11.3) [39].
The choice between upper and lower enteroscopy is guided by a preliminary noninvasive workup, including MR or CT enterography, followed by small bowel CE. If abnormalities are detected, then enteroscopy can facilitate the evaluation for a T cell malignant proliferation among jejunal ulcers, or perform histological follow-up of type I refractory celiac disease. Biopsies can aid in the detection of a clonal expansion or an abnormal phenotype of intraepithelial lymphocytes, suggesting the evolution toward type II refractory celiac disease or a T cell lymphoma. This surveillance is conducted yearly in refractory patients, and the choice between esophagogastroduodenoscopy, push enteroscopy, and deep enteroscopy is based primarily on the findings of small bowel capsule endoscopy [43]. Finally, upper enteroscopy allows one to obtain biopsy samples of suspicious lesions of the jejunum, such as deep ulcers or strictures, to rule out adenocarcinoma or T cell lymphoma (Fig. 11.4, Video 11.4).
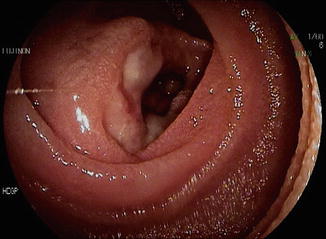

Fig. 11.4
Enteropathy-associated T cell lymphoma, presenting as an ulcerated jejunal stricture diagnosed by push enteroscopy
Contribution of Endoscopy in Other Malabsorption States
In addition to CD, other malabsorptive states can be evaluated with small bowel imaging. The causes of malabsorption can by roughly classified in three groups: (1) maldigestion, mainly linked to gastric and/or small bowel resection or pancreatic exocrine insufficiency; (2) mucosal noninfectious diseases, such as autoimmune enteropathy, common variable immunodeficiency, tropical sprue, and the recently described angiotensin II inhibitor-induced sprue; and (3) microbial causes, including a vast array of bacterial, viral, parasitic, or fungal infections occurring in immunocompetent or immunocompromised hosts, as well as small bowel bacterial overgrowth and Whipple’s disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








