Fig. 40.1
CD pathogenesis. Celiac disease ( CD) is a multifactorial disorder induced by gluten in genetically susceptible subjects. Several environmental factors (i.e., viral infections, alterations in microbiome composition, etc.) can contribute to trigger the disease by inducing epithelial stress in the intestinal mucosa, and the production of innate immune cytokines, such as interleukin 15 ( IL-15) and type-1 interferon ( type-1 IFN). Those cytokines contribute to create a pro-inflammatory environment that enhances dendritic cells activation. Undigested gliadin peptides ( purple circles) reach the intestinal lamina propria where they are deamidated by tissue-transglutaminase 2 ( TG2). Deamidation renders gliadin peptides more suitable to be presented by HLA-DQ molecules. Only dendritic cells expressing the CD-associated HLA-DQ2 and/or -DQ8 molecules ( orange) can present deamidated gliadin peptides ( green circles) to naïve CD4+ T cells ( red cells). Inflammatory dendritic cells ( purple) enhance the arise of a gluten-specific CD4+ T cell response, characterized by the production of high levels of pro-inflammatory cytokines such as interferon-gamma ( IFN-γ) and interleukin 21 ( IL-21). These cytokines are thought to contribute to induce a full activation of cytotoxic CD8+ intraepithelial lymphocytes ( IEL). The second hit required for the induction of a full activation of IEL is the expression of stress markers (i.e., MIC-A and IL-15) in intestinal epithelial cells. Fully activated CD8+ IEL are responsible for the intestinal epithelial destruction and the induction of villous atrophy in CD patients. Furthermore, gluten-reactive CD4+ T cells provide the required help to TG2-specific B cells, presenting TG2–gliadin complexes, in a hapten carrier-like manner and drive TG2-specific antibody production. NKG2D natural-killer group 2, member D, MIC macrophage inhibitory cytokine
The immunological reaction occurring in CD patients follows the ingestion of gluten-containing cereals: wheat, rye, and barley. Glutenins and gliadins, typical gluten components, are responsible for the viscosity and elasticity of the wheat dough [99, 100].Their high concentration of glutamine and proline residues (35 and 15 % of the total amino acid content) renders them highly resistant to GI enzymes [101]. Indeed, the lack of prolyl-endopeptidase activity in any of the human digestive enzymes prevents enzymatic attack of proline-rich domains in gluten proteins.Thus, at the end of a normal, full digestive process of gluten, many gliadin peptides remain undigested in the intestinal lumen. The mechanism through which such peptides reach the intestinal lamina propria is not entirely clear. Even though an increase in intestinal permeability has been shown in CD, a retro-transcytosis pathway has been described for secretory antibodies, potentially having a role also for gliadin transport [102].
Tissue-Tranglutaminase 2
In the subsequent steps in CD pathogenesis, the enzyme tissue transglutaminase 2 (tTG) has a pivotal role. tTG is a calcium-dependent transamidating enzyme that catalyzes covalent cross-linking of proteins. When located extracellularly in the presence of calcium, tTG is in an open and active form [103]; furthermore, an inflammatory environment leads to its constitutive activation [104]. tTG is the main autoantigen for CD: In addition, it has a crucial role in inducing posttranslational modification of gluten peptides. In fact, in the lamina propria, tTG mediates the conversion of glutamine into glutamic acid, introducing negatively charged residues into gliadin peptides that act as immunogenic epitopes binding to HLA-DQ molecules with relatively higher affinity, thus representing a prerequisite for a gluten-specific T cell response [105].
Autoantibodies
Genetic, mechanistic, and epidemiological data relate CD to other autoimmune disorders. One of the key diagnostic features of CD is the presence of serum anti-tTG IgA and IgG autoantibodies . The mechanisms leading to their production in CD are unclear. The upregulation and activation of tTG in inflamed tissues may generate additional antigenic epitopes by cross-linking or deamidating exogenous or endogenous proteins. However, the most accepted hypothesis for their formation, which explains also their dependence on dietary gluten, is that the enzyme cross-links itself to gluten during the substrate–enzyme interaction [106]. Once internalized, those complexes are processed and gluten epitopes bind HLA-DQ molecules of the B cell that may allow gluten-reactive CD4 T cells provide the required help to tTG-specific B cells in a hapten carrier-like manner and drive tTG-specific antibody production.
The Adaptive Immune Response in CD
CD originates as a result of both innate immunity and adaptive immunity activation. Adaptive response, characterized by the activation a gluten-specific CD4 T cell response in the intestinal lamina propria, is a key event in CD pathogenesis. Gluten contains a large number of peptides capable of stimulating T cells. Dendritic cells present negatively charged gliadin peptides through HLA-DQ2 or HLA-DQ8 molecules to naïve CD4 T cells, thus enhancing a T helper 1 inflammatory response, characterized by the production of high levels of interferon-γ (Fig. 40.1).
The gluten-specific CD4 T cell response is sustained by several cytokines, including IL-15 and IL-21. The expression of IL-21, enhanced by gluten, is very high in active CD patients, while it is downregulated in potential CD [107], supporting its role in the induction of tissue damage. It acts in synergy with IL-15 [108] promoting interferon-γ production and enhancing cytotoxic CD8 T cells proliferation and survival; however, the mechanism initiating its production in CD is unclear.
The Innate Immune Response
As mentioned, innate immune activation is also necessary for the development of CD. There is in fact evidence that in addition to the immunodominant epitopes, which as, discussed, are presented to CD4 naïve T cells, gliadin also contains fragments which are able to enhance epithelial stress and induce an innate immune effect [109–111]. The best-known fragment is the peptide 31–43 (P31–43) of α-gliadin, which is able to upregulate major histocompatibility complex (MHC) class I-related molecules (MICs) [112] to activate the mitogen-activated protein (MAP) kinase pathway and induce apoptosis in intestinal epithelial cells. P31–43 induces cell proliferation and actin cytoskeleton rearrangements in in vitro and ex vivo models [109–111, 113, 114]. The proliferative response elicited by P31–43 in CD mucosa involves epidermal growth factor (EGF)/IL15 cooperation. Of note, a constitutive activation of the EGF receptor (EGF-R)/extracellular signal-regulated kinases (ERK) pathway has been found in celiac patients [110] potentially representing a predisposing condition to the damaging effects of gliadin.
Of interest, P31–43 is able to impair actin cytoskeleton in healthy subjects, suggesting that it can act on similar pathways altered in CD cells. In addition, it enhances the expression of IL-15, a key innate cytokine involved in CD pathogenesis, as it contributes actively to enhance the expression of activating natural killer cells (NK) receptors (i.e., NKG2D) on IELs and impair the T regulatory cells function in celiac patients [115, 116].
IELs Activation and the Induction of Tissue Damage
The tissue damage typical of CD, characterized by villous atrophy and crypt hyperplasia, is due to a profound remodeling of the small intestinal architecture and is mainly mediated by cytotoxic IELs. IELs represent a heterogeneous population including TcRα/β CD8 cells NK-like cells, and TcRγ/δ cells.
As evident in potential CD patients, the gluten-specific T cell adaptive immune response alone can occur even in the absence of villous atrophy, suggesting that other signals are required to induce tissue damage [98]. Indeed, the full activation of IELs and acquisition of their cytotoxic killing phenotype requires also stimuli from the epithelial compartment. Increased expression of stress molecules, such as nonclassical MHC class I molecules (i.e., MIC and HLA-E), has been shown in intestinal epithelial cells of active CD patients, as part of the innate stress response induced by gluten or by other environmental triggers. MIC and HLA-E are ligands for NKG2D and CD94, activating NK receptors that are upregulated on IELs in CD patients and whose expression is enhanced by IL15. Activation of IELs, with increased Fas ligand expression, results in epithelial cell apoptosis and villous atrophy via interactions with Fas on intestinal epithelial cells.Altogether, the end result of these various events is to lead to IEL infiltration and the resulting tissue destruction, characterized by crypt hyperplasia and villous atrophy.
These changes occur in a continuum, going from normal to a complete flattening of the villi in a slow progression. Marsh [117] described in great detail such progression and his description is also utilized in the pathology reports from duodenal biopsies. Thus, the intestinal damage seen in CD is described as follows:
Type 0 or pre-infiltrative stage (normal);
Type 1 or infiltrative stage (increased IELs);
Type 2 or hyperplastic stage (type 1 + hyperplastic crypts);
Type 3 or destructive stage (type 2 + villous atrophy of progressively more severe degrees, denominated 3a—partial atrophy, 3b—subtotal atrophy, and 3c—total atrophy).
Clinical Presentations
The inflammatory changes described in the previous section, resulting in the most advanced cases in severe villous atrophy, lead to a wide variety of clinical presentations. While GI manifestations are understandably present and in many cases prominent, CD goes well beyond the GI tract, so that basically all systems and organs can be involved. GI signs and symptoms due to malabsorption , such as diarrhea and abdominal pain, are very common and easily lead to evaluation for CD, but they are by no means universally present. In fact, there is evidence [118–122] that CD presentation in children and teenagers has substantially changed over time, moving from a malabsorptive disorder causing GI symptoms and malnutrition to a more subtle condition causing a variety of extraintestinal manifestations (see Table 40.1). Thus, it is understandable that the term “typical,” reserved for the GI manifestations of CD, is quickly becoming obsolete, as the extraintestinal manifestations are now so prevalent they are no longer to be referred to as “atypical” [123]. It is indeed this variety of presentations, and the fact that CD may also be entirely asymptomatic, that is responsible for the dismal rate of diagnoses around the globe.
Table 40.1
Clinical presentations of celiac disease according to age most commonly involved
Type | Sign or symptom | Age most commonly involved |
|---|---|---|
Gastrointestinal | Abdominal pain, bloating | All ages |
Diarrhea | All ages | |
Vomiting | Infancy | |
Anorexia | Infancy to early childhood | |
Constipation | Child to adolescent | |
Failure to thrive | Infancy and early childhood | |
Weight loss | Child to adult | |
Recurrent intussusception | Infancy to early childhood | |
Hypertransaminasemia and other liver issues | All ages | |
Extraintestinal | Sad mood | Infancy to early childhood |
Delayed puberty | Adolescent | |
Short stature | Child to adolescent | |
Iron-deficient anemia | Adolescent to adult | |
Dermatitis herpetiformis | Adolescent to adult | |
Dental enamel defects | Child to adult | |
Aphthous ulcers | Child to adult | |
Fatigue | Adolescent to adult | |
Arthritis | Adolescent to adult | |
Osteopenia | Adolescent to adult | |
Osteoporosis | Adult | |
Psychiatric disorders | Adolescent to adult | |
Idiopathic seizures | Child to adult | |
Headaches, migraines | Adolescent to adult | |
Numbness/neuropathy | Adult | |
Cerebellar ataxia | Adult | |
Unexplained infertility (in women) | Adult |
Table 40.1 reports the main clinical presentations of CD, with their prevalent age distribution. As it can be seen, the clinical manifestations can be protean, thus making the diagnosis not obvious in most cases. When CD has its onset in infancy and very early childhood, the GI manifestations prevail and can be quite aggressive, resulting in a clinical picture of malnutrition and failure to thrive, often associated with a protein-losing enteropathy. Subsequently, however, the onset may be more subtle, and more extraintestinal manifestations become common .
GI Manifestations
Abdominal pain and distention is probably the most common symptom of patients diagnosed with CD worldwide; in Canadian children, it has been reported in as many as 90 % [124]. Chronic or intermitted diarrhea, characterized by bulky, foul-smelling, greasy stool, is a very common symptom in children with CD. Its occurrence, however, is progressively becoming less frequent than in the past. Counterintuitively, long-standing and occasionally severe constipation can be the presenting manifestation in a significant amount of patients, children, as well as adults [125]. Constipation appears to be related to a well-documented delay in oro-cecal transit time [126, 127], possibly caused in part by disturbed upper GI motor function [128] . Other presenting symptoms related to the GI tract are vomiting (especially in infants and toddlers), weight loss, or failure to thrive leading—particularly in cases of delayed diagnosis—to severe malnutrition and cachexia. However, it should be noted that children with CD can also be overweight or obese , as well documented in the literature, (reviewed in [129]) so that the absence of malnutrition should by no means rule out the possibility of CD. More rarely, other disorders such as acute electrolyte disturbances, hypotension, and lethargy can accompany the clinical picture [130]. Recurrent intussusception, an uncommon but important GI sign, was first described in children in 1997 [131], and it is now well recognized as an event occurring more frequently in CD children before their diagnosis than in control populations [132].
Mild elevation of liver transaminases is well described in pediatric CD. A recent meta-analysis [133] revealed that this sign is presented by 36 % of children with CD, while 12 % of children with mild unexplained hypertransaminasemia have CD. Of note, a gluten-free diet (GFD) normalized transaminase levels in 77–100 % of patients with CD within 4–8 months .
Extraintestinal Manifestations
Anemia in celiac children can be the end result of several different, and sometimes combined, causes; however, the single most common type of anemia is due to iron deficiency.
IDA has in fact been reported in between 12 and 69 % of newly diagnosed celiac cases [10–12, 122, 134]. Even when asymptomatic, CD can lead to IDA; in a large series of patients with subclinical CD, IDA was indeed found in almost half of the patients, with adults having a higher incidence than children: 46 versus 35 % [10]. The pathogenesis of iron deficiency in celiac seems to be straightforward; in fact, iron is absorbed in the duodenum and proximal jejunum, areas that are typically most affected in florid CD. Thus, most cases result from an impaired absorption of iron. The higher prevalence of anemia in celiac patients with an atrophic mucosa (Marsh 3) compared to those with mild enteropathy (Marsh 1 or 2) recently found in a study conducted in Italy on a large number of patients [135] provides indirect support for this.
Dermatitis herpetiformis (DH) is considered the skin presentation of CD. Rare in children, DH affects mostly teenagers and adults who present symmetrical, pruritic blisters followed by erosions, excoriations, and hyperpigmentation. The most commonly involved sites are the elbows (90 %), knees (30 %), shoulders, buttocks, sacral region, and face. Itching of variable intensity, scratching, and burning sensation immediately preceding the development of lesions are common [136, 137]. The diagnosis rests on a combination of clinical, serological (same autoantibodies utilized for CD), and histological criteria showing the typical IgA deposits in skin biopsies [138, 139]. Its treatment is based on a strict GFD. Dapsone and/or other drugs should be used during the period until the GFD is effective [138].
Dental enamel hypoplasia is more common in patients with CD compared to the general population [26], and in CD it has been reported with a prevalence ranging from 10 to 97 %. It appears to be more prevalent in children, compared with adults with CD and is thought to be secondary to nutritional deficiencies and immune disturbances during the period of enamel formation in the first 7 years [27].
Aphthous ulcers too can be present in children and adults with CD and they often regress once the patients are on a GFD [28].
Low bone mineral density is found in the vast majority of newly diagnosed patients, and in some cases it is advanced to osteoporosis and can be associated with fractures. The etiology of such bone alterations in CD is multifactorial, thought to be mostly secondary to the combination of intestinal malabsorption and chronic inflammation. At diagnosis, approximately one-third of adult CD patients have osteoporosis, one-third have osteopenia, and one-third have normal bone mineral density [29]. While children with CD, once on a GFD, seem to be able to improve their bone mineral density more fastly, adult patients’ bone mineral density was found to be significantly lower than expected for the normal population, not just at diagnosis, but even after 1 or 2 years of GFD [140]. Thus, there is a risk for suboptimal peak bone mass acquisition and a retarded growth in CD children, as the bone density increases until the end of puberty , when it reaches its peak value: if normal peak bone mass is not achieved, the individual is at a higher risk for developing osteoporosis [141].
Joint involvement, though not too common, has been described in children and adults with CD and it is suggested that patients presenting with unexplained articular manifestations be tested for CD [142].
Children with CD have a slightly increased frequency of neurological symptoms compared to controls. These include headache [143], peripheral neuropathy [144], and seizures; ataxia is described only in adult cases [145]. The prevalence of epilepsy in CD children also appears to be slightly higher than expected, around 1 % [146]. Seizures are often generalized tonic–clonic, but partial and occasionally absence seizures are also reported [146, 147]. A recent epidemiologic study of nearly 29,000 subjects with CD and 143,000 controls found that CD increased the risk of epilepsy, including in children, by 1.4-fold [148]. In some patients with CD and epilepsy refractory to antiepileptic drugs, seizures have been controlled with a GFD [149, 150].
Relatively common in adolescents are also psychiatric issues: anxiety, often with recurrent panic attacks, hallucinations, depression, leading to a slightly higher prevalence of suicidal behavior in celiac patients [30]. Interestingly, there is some evidence that the GFD may help in alleviating depression in celiac adolescents [31].
Disease Associations
A series of recent large-scale epidemiological investigations, mostly, but not solely, conducted in Sweden, have also revealed a growing number of associated conditions that can occur with CD, although in most cases the reasons for such associations and the clinical relevance of them remain unclear. Among the conditions with increased prevalence in CD, in most cases related to adult patients, are: chronic obstructive pulmonary disease [151], ischemic heart disease [152], urticaria [153], eosinophilic esophagitis and gastroesophageal reflux disease [154], pancreatitis [155], hemochromatosis [156], cataracts and uveitis [157, 158], idiopathic dilated cardiomyopathy [159], nephrolithiasis [160], end-stage renal disease [161], eating disorders [162], primary hyperparathyroidism [38] (a risk however subsiding on GFD), adrenal insufficiency [33], systemic lupus [163], cataract [40], and endometriosis [39].
In addition, the offspring of celiac mothers appear to also be at an increased risk for a number of adverse events, including congenital malformations [164].
On the other hand, a reduced risk for ovarian and breast cancer has been reported for celiac women [165].
IgA deficiency has also been associated with CD. In fact, about 8 % of IgA-deficient children are celiac [166] and about 2 % of CD children are IgA-deficient [167]. While clinically not relevant, this association is important in the strategy of screening for CD (see below).
Autoimmune Conditions
A strong association has also been shown between CD and autoimmune disorders, thought to be mostly due to a shared genetic component in the HLA region. The best described association is with type 1 diabetes mellitus (T1DM), where a prevalence of approximately 10 % of CD is found [32, 168]. About two-thirds of children diagnosed with CD after T1DM onset have elevated levels of celiac antibodies at the time of T1DM diagnosis or within the first 24 months; however, an additional 40 % of patients develop CD a few years after diabetes onset [169], and even adults with a long history of T1DM show a progressively higher prevalence of CD [170]. Thus, it is recommended that T1DM children be repeatedly tested for CD. Of note, it has been shown that the presence or absence of GI symptoms in children with T1DM has no predictable value for biopsy-confirmed CD or not [168]. Clearly, once diagnosed with CD, children with T1DM need to follow a GFD. Its effects on diabetic control are however unclear: In fact, glycemic control, both in children with or without malabsorption, has been found either improved or unaffected [171].
The issue of whether the onset of autoimmune disorders in CD patients is favored by the ingestion of gluten remains controversial. An increased prevalence of autoimmune disorders was found in parallel with the increasing age at diagnosis of CD [172], suggesting that prolonged exposure to gluten may favor the onset of autoimmune conditions; however, these results have not been reproduced by subsequent investigations [173, 174], leaving the question unresolved [175].
Diagnosing CD
Given the many various manifestations of CD, in children as well as adults, clearly the first requisite for diagnosis is a high degree of suspicion. Table 40.2 lists the circumstances that demand testing for CD .
Table 40.2
Subjects to be screened for CD
All conditions listed in Table 40.1 |
First-degree relatives of celiac patients |
IgA deficiency |
Autoimmune conditions |
Type 1 diabetes |
Autoimmune thyroiditis |
Autoimmune hepatitis |
Addison’s disease |
Chromosomal disorders |
Down syndrome |
Turner syndrome |
Williams syndrome |
The availability of very sensitive and specific autoantibodies in the IgA subclass, generated in the small intestinal mucosa and detectable in the serum, such as the tTG-IgA or anti-endomysium IgA (EMA-IgA) [179] has given the physician a powerful screening tool. In addition, another class of antibodies has also been found valuable in screening (especially in very young children when tTG-IgA could be negative [180, 181]) and in following up patients with CD : the anti-DGP , both -IgA and -IgG antibodies produced against the gliadin peptides after they have been modified by the tTG [182]. Currently, it is universally recommended [33, 179, 183] that tTG-IgA and total serum IgA be the first line of screening, due to the very elevated sensitivity of this test. Total serum IgA needs to be added in order to ascertain that the individual is able to produce tTG-IgA: In fact, celiac patients who happen to be IgA-deficient may have a false-negative tTG-IgA. In these circumstances, both tTG-IgG [184–186] and DGP-IgG [187] can be usefully checked as markers of CD.
In 1990, the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) published diagnostic criteria [188] that have been universally applied for over 20 years, both in pediatric age and in adults. While reducing the number of biopsy procedures needed for a firm diagnosis from three of the original guidelines (initial one showing typical changes, followed after a year on GFD by a second one documenting healing, and finally by a third one after gluten challenge to show relapse) to only one, they still called for the indispensable role of documenting the flattening of small-intestinal mucosal villi in patients with a consistent history and laboratory findings. In 2012, an ad hoc task force of the same society published revised criteria [1] and produced an evidence-based algorithm that allowed the physician to skip the duodenal biopsy under certain circumstances, namely, in children and teenagers showing a genetic asset and a history compatible with CD, a very elevated titer (more than ten times the upper limit of normal) of tTG-IgA, along with a positive EMA titer. While this simplified approach appears certainly valid, as it possesses a positive predictive value close to 100 %, one needs to apply it with great care. In fact, children with GI complaints diagnosed without the endoscopy may have additional disorders that would go undiagnosed by skipping this procedure.
Figure 40.2 is an algorithm (based in part on the ESPGHAN guidelines [1]) that summarizes the diagnostic steps for a child or teenager suspected of CD. Again, Table 40.2 lists the presentations that require screening. The subject with normal serum IgA and normal tTG-IgA do not need to be considered celiac, given the high sensitivity of the test; however, since it would appear that in the infant and toddler tTG-IgA may not be as sensitive as in later ages, DGP-IgA and DGP-IgG can be measured additionally. Furthermore, in the presence of a strong clinical suspicion in the very young child, it would be appropriate to still proceed with an esophago-gastro-duodenoscopy (EGD) with biopsy even if celiac serology is negative.
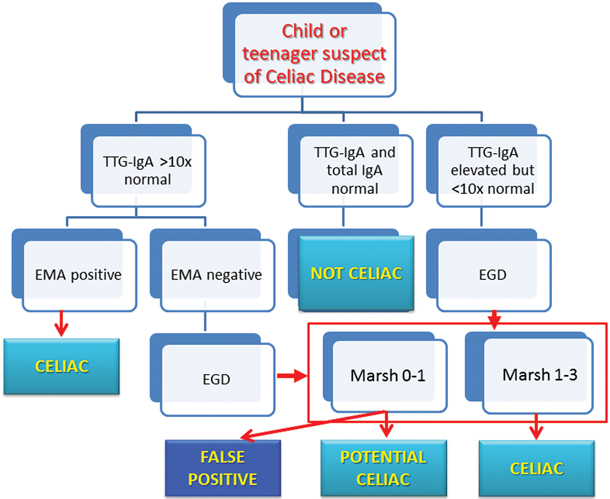

Fig. 40.2
Suggested diagnostic algorithm
If the subject has positive tTG-IgA, then the titer becomes important. In fact, as mentioned, with titers that are more than ten times the upper limit of normal range, the EMA titer must be checked and if it too is clearly positive, then the diagnosis of CD can be considered definitive. On the contrary, the EGD would be necessary if the EMA prove negative, or in all cases where the tTG-IgA increase does not reach the threshold of > 10× normal. It is important to notice that it is recommended [1, 183, 189] that at least four biopsies be taken from the distal duodenum, and also one or two from the bulb, otherwise the diagnostic yield may be jeopardized by the occasional patchy duodenal lesions.
The interpretation of the pathology of the duodenal biopsies guides the final diagnosis: while pathology changes showing lesions of Marsh type 2–3, in the presence of positive serology, confirm the diagnosis, caution must be exerted for findings of Marsh type 1, especially when not supported by positive serology. In fact, such increased presence of IELs (“lymphocytic duodenosis”) has been found to be due to celiac in no more than 16–39 % of cases [190, 191]. Thus, additional conditions (see Table 40.3) must be carefully looked at before concluding for its association with CD.
Table 40.3
Conditions causing lymphocytic duodenosis (Marsh type 1 changes)
Celiac disease |
H. pylori gastritis |
Small-bowel bacterial overgrowth |
NSAID and other drugs |
Immune dysregulation |
Crohn’s disease |
Food protein-induced enteropathy |
Infections |
As for those with a Marsh type 0, the call is even more delicate. In fact, the patient can be defined as “potential” if tTG-IgA are elevated > 10× and/or EMA titer is positive; but with tTG-IgA increased less than ten times and a negative EMA, then the possibility of a false-positive tTG-IgA must be considered. This occurrence is especially common, for unclear reasons, in children with T1DM, where tTG-IgA have also been found to spontaneously normalize [192]. Such patients should therefore remain on a gluten-containing diet and be carefully monitored.
More complex is the decision about the need for a GFD for true “potential” celiac children and adolescents who are asymptomatic. The literature shows in fact variable percentages (between 30 and 60 %) of evolution into full-blown CD when they are left on gluten [193–195], including the possibility of some eventually becoming serologically negative. It seems in fact that potential CD patients show a low grade of inflammation that likely could be due to active regulatory mechanisms preventing the progression toward a mucosal damage [196]. In essence, while research will eventually allow us to predict which patients would develop full-blown CD if left on gluten, currently we do not have this capacity, so that the decision on whether or not to put potential celiac patients on a GFD must be taken with great care, on an individual basis, and be properly agreed upon by the family.
Complications
Refractory CD
Better known by the older terminology as “refractory sprue,” refractory CD is a very severe form of CD that does not respond to a GFD, in spite of normalization of celiac serology [197]. Refractory CD almost exclusively occurs in adults or elderly patients who have been suffering from malabsorptive symptoms for a long time prior to being diagnosed. This condition is further classified into type I and type II on the basis of gamma chain T cell clonal rearrangement and aberrant T cell phenotypes. Type II refractory CD is the most aggressive form, leading to the most feared complication of CD: the enteropathy-associated T cell lymphoma (EATL). As a consequence, refractory CD results in an increased mortality rate, with a 5-year survival rate of 80–96 % for patients with type I refractory CD and 44–58 % for type II cases [197]. When examining the survival rate of those patients with type II refractory CD who developed EATL, the survival rate at 5 years was a dismal 8 % [198]. New treatment modalities, including autologous stem cell transplant, are becoming available for this aggressive condition [199, 200].
Increased Mortality Rate
Aside from the risks related to refractory CD, evidence is mounting that unrecognized, and hence untreated, CD may carry a risk for increased mortality rate [201]. There appears to be a positive correlation between diagnostic delay and/or insufficient compliance with the diet and decreased life expectancy, which has been documented in a large retrospective study in Italy [202]. More recently, increased mortality has also been reported in undiagnosed patients (based on elevated serum tTG-IgA) in the USA [203] and in Europe [204–206].
All of the evidence therefore strongly emphasizes the importance of an aggressive strategy for early detection and treatment of patients with CD.
Treatment
CD is a lifelong condition and a strict GFD is currently the only available treatment. While the exact amount of gluten that can be tolerated daily by CD patients is unknown and is likely to be subject to a wide interindividual variability, there is evidence that no patient would react to a daily dose of up to 10 mg, while the majority would at 100 mg or above [207]. In practice, many patients, and especially older children and adults, do not present any symptom after inadvertent gluten ingestion or sporadic intentional consumption of gluten-containing products. In addition, dietary compliance assessment is not always an easy task, since serology often fails to reveal slight transgressions. Therefore, a periodical follow-up is needed and the importance of a lifelong diet should be constantly reinforced, especially to asymptomatic patients. The dietary restriction must include wheat, rye, barley, and their derivatives such as triticale, spelt, and kamut. Other cereals such as rice, corn, maize, and buckwheat are perfectly safe for CD patients and can be used as wheat substitutes. Oats were included in the first group of toxic cereals, but later evidence has conclusively shown that they are tolerated by the vast majority of patients, provided the oats-based products are manufactured in plants that can guarantee absence of any possible cross-contamination with flours based on wheat, rye, or barley. In any case, introducing them with prudence is recommended in order to recognize any adverse effect.
Gluten withdrawal is necessary to shut down the inflammatory response arising in the gut. However, whether a GFD could prevent the development of associated autoimmune conditions or complications, such as malignancies, is still debated [175]. As discussed previously, since the benefits of GFD in terms of prevention of complications and comorbidities in patients with potential CD are still unclear, it remains controversial whether such dietary regimen should be proposed to all of them, particularly those who are asymptomatic.
Future Potential Therapeutic Strategies
Following a lifelong strict dietary regimen is not an easy task for many patients, especially teenagers and young adults who may not easily accept limitations to their social life. On the other hand, there is a subgroup of CD patients (i.e., refractory CD) who fail to respond even to a strict GFD, thus reinforcing the concept that GFD could not be considered a cure for all CD patients. Importantly, a variety of commercial food products and some safe cereals can be cross-contaminated with unsafe cereals as the result of mixing during transportation and processing. Hence, there is a requirement for alternative approaches to treat CD.
A first approach may consist in creating new varieties of wheat that are safe for CD patients. The identification of the full sequences of immune-stimulating peptides may lead to the production of wheat varieties lacking biologically active peptide sequences through breeding programs and/or transgenic technology. Large-scale cultivars where these cereals could replace the current toxic varieties could reduce the risk of possible cross-contamination. However, it is evident that this process will take a long time to be developed and would still require for the patients the need to carefully select such products.
Recent progresses in understanding the cellular and molecular basis of CD have helped in identifying several possible therapeutical targets, from already available molecules that can find a new application in this disease to new drugs that may be developed. Strategies vary from gliadin-degrading enzymes that would allow the occasional consumption of gluten-containing products to the more ambitious attempt to reestablish immunological tolerance toward gluten using a peptide-based “vaccine.”
Given the high content in proline residues, gliadin peptides are highly resistant to proteolysis, thus favoring the accumulation of long fragments within the intestinal lumen. Exogenous proline and/or glutamine-specific proteases-based drugs and/or dietary supplements (i.e., ALV003 (glutenase Alvine 003) and AN-PEP (alanyl (membrane) aminopeptidase)) facilitate gluten digestion and T cell epitopes destruction [208], thus representing a useful “adjunctive” tool for CD under a GFD, in particular situations where there is a risk of cross-contamination. Another possible gluten detoxifying strategy is represented by gluten-sequestering polymers (i.e., hydroxyethyl methacrylate-co-styrene sulfonate; HEMA-co-SS) that bind gluten fragments, reducing their effects on the intestinal mucosa [209].
A pharmacological reduction of intestinal permeability may contribute to reduce the load of gliadin peptides reaching the intestinal lamina propria. In this perspective, larazotide (AT-1001), a zonulin antagonist, is undergoing clinical trials in CD patients. Furthermore, inhibition of Rho kinase (RhoA or ROCK), two molecules regulating cytoskeleton and tight junction structure, has been proposed as future potential target [210].
Furthermore, the identification of specific epitopes may also provide the basis for immunomodulation by antigenic peptides. In this regard, the most ambitious and promising therapeutic intervention for CD patients is represented by a vaccine based on a set of gliadin immunodominant peptides recognized selectively by HLA-DQ2 and that should promote the induction of tolerance to gluten through a peptide-based desensitization [211] .
Other promising approaches include preventing gliadin presentation to T cells by blocking HLA-binding sites [212] or use of tTG inhibitors [213], thus impairing the adaptive immune response activated by gliadin peptides. Any immunomodulatory approach will be required to have a high safety profile to be a valuable substitute to the GFD.
Anti-IL-15 monoclonal antibodies, already tested in rheumatoid arthritis and psoriasis, have been proposed for refractory CD, where the expansion of IELs is dependent on this cytokine. Blocking NKG2D to prevent full activation of IELs has also been proposed as a further potential therapeutic target in CD patients [214]. Again, security profile, side effects, and compliance will need to be taken into account before any of those treatments could replace GFD.
References
1.
Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136–60.PubMed
2.
Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62(1):43–52.PubMedCentralPubMed
3.
Gee SJ. On the celiac affection. St Barth Hosp Rep. 1888;24:17–20.
4.
van Berge-Henegouwen GP, Mulder CJ. Pioneer in the gluten free diet: Willem-Karel Dicke 1905–1962, over 50 years of gluten free diet. Gut. 1993;34(11):1473–5.PubMedCentralPubMed
5.
Shiner M. Coeliac disease: histopathological findings in the small intestinal mucosa studies by a peroral biopsy technique. Gut. 1960;1:48–54.PubMedCentralPubMed
6.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








