Detrusor injection of botulinum toxin (BTX) has shown great promise in the treatment of neurogenic detrusor overactivity (NDO) refractory to conservative therapy. Despite a paucity of prospective evidence, there exists a growing consensus that BTX injection therapy is a well-tolerated, low-risk therapy. Injections result in substantial subjective improvement in continence and quality of life. Moreover, assessment of urodynamic parameters demonstrates objective changes: (1) an increase in maximum cystometric capacity; (2) when applicable, a reduction in maximal detrusor voiding pressures; and (3) an increase in bladder compliance in cases where baseline bladder compliance measures were abnormal. While BTX bladder injection offers both objective and subjective measures of incontinence control, treatment duration is limited by the gradual reinnervation of injected tissue over an approximately 6- to 9-month interval. However, repeat injection cycles do appear to achieve similar levels of efficacy. The objective of this review is to provide a focused summary of the current body of literature, investigating the safety and efficacy of bladder BTX injection in patients with NDO.
The overactive bladder (OAB) condition is a symptom complex characterized by urinary urgency with or without urge urinary incontinence (UUI), and is often associated with frequency and nocturia. Overall, OAB prevalence in the United States and European adult population is estimated to be 11.8%. OAB is not specific to any one condition. However, based on clinical convention; OAB may then be further classified as neurogenic or idiopathic. When OAB occurs in association with a known underlying neurologic pathology such as spinal cord injury (SCI) or multiple sclerosis (MS), it is classified as neurogenic OAB. When there is no evidence of any identifiable neurologic disorder, it is classified as idiopathic OAB.
OAB is a clinical diagnosis. By contrast, detrusor overactivity (DO) describes the common urodynamic testing observation in this population, whereby involuntary detrusor contractions become evident during the bladder-filling phase. For the purposes of this discussion of neurogenic bladder dysfunction, the authors use the term neurogenic detrusor overactivity (NDO).
Research demonstrates the significant impairment to quality of life (QOL) by OAB as well as its added burden of increasing health care costs. While the OAB condition clearly affects QOL for all sufferers, the subset of patients with NDO can be even more disadvantaged by virtue of their neurologic deficits.
Moreover, neurogenic bladder dysfunction has the distinct potential to cause long-term renal failure. The neural and/or myogenic deficits may gradually compromise the bladder’s storage function, otherwise known as bladder compliance. Such insidious, asymptomatic deterioration of the neurogenic bladder’s compliance reflects a gradual loss of the lower urinary tract’s ability to store increasing volume at the same low ambient pressure. With the ensuing increase in intravesical storage pressure, the lower urinary tract often responds by provoking random urethral leakage as a means to vent a high-pressure chamber. However, in some instances where the system fails to satisfactorily vent the high-pressure chamber, the sustained high pressures transmit upstream to the renal pelvis. Over time, this sustained high-pressure transmission to the renal pelvis causes deterioration of glomerular filtration and eventual renal failure. This well-characterized pathophysiologic process poses a dangerously asymptomatic phenomenon for the NDO population.
Typically, first-line therapy for idiopathic OAB is conservative in nature, encompassing behavioral techniques, pelvic physiotherapy, and antimuscarinic pharmacotherapy. While conservative therapies are likewise reasonable for first-line management of NDO, the chances of successful incontinence control are greatly hindered by patient disabilities and the underlying neurologic defects within the complex micturition circuit. In other words, a significant fraction of NDO patients derive insufficient bladder control with conservative treatment strategies. Moreover, this patient population disproportionately suffers more overall QOL impairment with their lower urinary tract dysfunction.
A growing body of evidence suggests that intravesical injection of botulinum neurotoxin (BTX) is an efficacious, minimally invasive alternative to more traditional surgical therapies (such as bladder augmentation or urinary diversion) in patients with NDO who are intolerant or refractory to pharmacotherapy. Alternative intravesical agents currently being evaluated include antimuscarinic agents (oxybutynin, atropine), local anesthetics (lidocaine, bupivacaine), and vanilloids (capsaicin, resiniferatoxin), but randomized, placebo-controlled evidence demonstrating safety and efficacy is currently lacking. The objective of this review is to provide a focused summary of the current body of literature investigating the safety and efficacy of bladder BTX injection in patients with NDO.
Mechanism of action
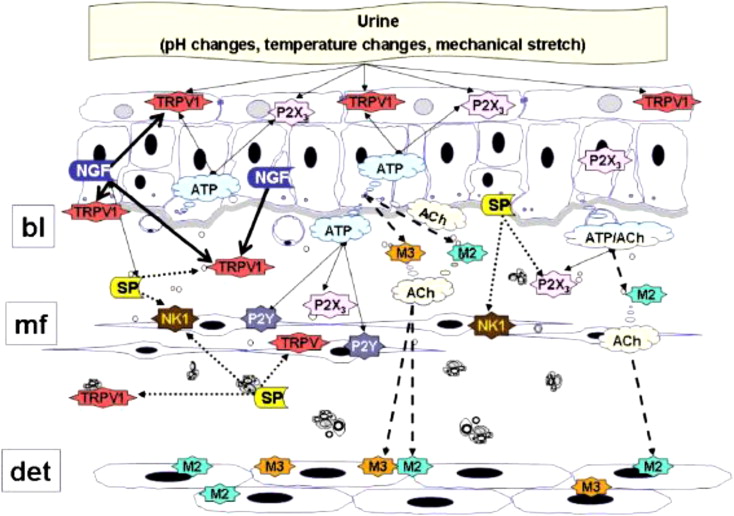
Botulinum toxin, produced by the gram-positive anaerobic bacterium Clostridium botulinum and first isolated by van Ermengem in 1897, is among the most potent biologic neurotoxins known to man. Structurally composed of a 150-kDa amino acid di-chain molecule consisting of a light (50 kDa) and heavy (100 kDa) chain linked by a disulfide bond, BTX’s primary mechanism of action is inhibition of acetylcholine release at the presynaptic cholinergic junction. BTX’s high selectivity for cholinergic synapses results in targeted blockade of cholinergic transmission, which when targeted to striated muscle induces muscle paresis. More specifically, BTX reduces type Ia/II intrafusal muscle fiber afferent conduction, affecting the spinal stretch reflex and decreasing muscle tone and contractility without affecting muscle strength. Investigators have postulated recently that, in addition a direct effect on detrusor motor innervation, BTX may also affect afferent nervous transmission via the inhibition of acetylcholine, adenosine triphosphate (ATP), glutamate, nerve growth factor, and substance P, and a reduction in the axonal expression of nerve capsaicin and purinergic (P2X) receptors. Thus, it seems likely that BTX also modulates intrinsic bladder reflexes, resulting in central desensitization and a decrease in urgency ( Fig. 1 ). Duration of effect is likely multifactorial as well; mechanisms including chemical denervation followed by axonal regeneration as well as functional motor suppression through neurotransmitter inhibitory effects have been proposed.
Serotypes, toxicities, and commercial preparations
Seven BTX serotypes have been isolated, 2 of which (BTX-A and BTX-B) have been investigated in treating bladder dysfunction. The most commonly investigated serotype worldwide, BTX-A received Food and Drug Administration (FDA) approval for therapeutic treatment of strabismus and blepharospasm in 1989, cervical dystonia in 2000, and cosmetic treatment of glabellar wrinkles in 2002. In a recent review of all adverse events (AE) reported to the FDA since BTX-A was licensed in 1989, Cote and colleagues described 217 serious AE, including 28 deaths. All occurred during therapeutic administration but the investigators concluded that it was impossible to determine a causal relationship between the reported mortalities and BTX administration. Additional AE included dysphagia (12%), muscle weakness (6%), allergic reaction (5%), and flu-like syndrome (5%). During cosmetic use, the investigators reported primarily nonserious AE and no deaths, most commonly lack of effect (63%), injection site reaction (19%), and ptosis (11%). Although the FDA has not yet approved BTX for urologic use in the United States, BTX is currently being investigated for urologic indications in clinical trials worldwide. At present, BTX is available as 3 major commercial preparations: Botox (BTX-A; Allergan, Irvine, CA, USA), Dysport (BTX-A; Ipsen, Slough, UK), and Myobloc (BTX-B; Solstice Neurosciences, San Diego, CA, USA). Each of these preparations has different dosages, safety profiles, and efficacy profiles, and cannot be used interchangeably. In the only head to head comparison to date, Grosse and colleagues performed an open-label, observational case-control study comparing a single treatment session of Dysport (500 U, 750 U, 1000 U) and Botox (300 U), finding no differences in therapeutic parameters between groups.
Serotypes, toxicities, and commercial preparations
Seven BTX serotypes have been isolated, 2 of which (BTX-A and BTX-B) have been investigated in treating bladder dysfunction. The most commonly investigated serotype worldwide, BTX-A received Food and Drug Administration (FDA) approval for therapeutic treatment of strabismus and blepharospasm in 1989, cervical dystonia in 2000, and cosmetic treatment of glabellar wrinkles in 2002. In a recent review of all adverse events (AE) reported to the FDA since BTX-A was licensed in 1989, Cote and colleagues described 217 serious AE, including 28 deaths. All occurred during therapeutic administration but the investigators concluded that it was impossible to determine a causal relationship between the reported mortalities and BTX administration. Additional AE included dysphagia (12%), muscle weakness (6%), allergic reaction (5%), and flu-like syndrome (5%). During cosmetic use, the investigators reported primarily nonserious AE and no deaths, most commonly lack of effect (63%), injection site reaction (19%), and ptosis (11%). Although the FDA has not yet approved BTX for urologic use in the United States, BTX is currently being investigated for urologic indications in clinical trials worldwide. At present, BTX is available as 3 major commercial preparations: Botox (BTX-A; Allergan, Irvine, CA, USA), Dysport (BTX-A; Ipsen, Slough, UK), and Myobloc (BTX-B; Solstice Neurosciences, San Diego, CA, USA). Each of these preparations has different dosages, safety profiles, and efficacy profiles, and cannot be used interchangeably. In the only head to head comparison to date, Grosse and colleagues performed an open-label, observational case-control study comparing a single treatment session of Dysport (500 U, 750 U, 1000 U) and Botox (300 U), finding no differences in therapeutic parameters between groups.
Rationale for treatment
Since the 1980s, clinicians have been using BTX to treat neurologic disorders, such as blepharospasm, strabismus, focal dystonias, muscle spasms and spasticity, axillary hyperhidrosis, and achalasia. The initial application of BTX-A to the lower urinary tract targeted patients with detrusor external sphincter dyssynergia (DESD). BTX-A was injected into the urethral sphincter with the intention of weakening the urethral striated musculature enough to reduce urethral pressures, and in turn, to decrease bladder voiding pressures and post-void residual volumes (PVR). However, over the past decade the off-label use of BTX in treating detrusor overactivity (both neurogenic and idiopathic) has become a source of significant interest among urologists. Demonstrating posttreatment reduction in detrusor pressures during voiding and involuntary contractions has provided evidence of BTX’s effect on detrusor motor innervation, and patient-reported reduction in sensation of urgency lends support to a dual afferent mechanism of action as well.
With current investigations expanding the utility of BTX even further to include interstitial cystitis, pelvic floor dysfunction, and prostatic applications; there is currently a wide variation in reported dosages, injection techniques, and follow-up protocols being used. This pattern has led to current efforts to create evidence-based guidelines governing BTX use. However, it is clear that the dosage, number, and site of injection should be tailored to the individual needs of each patient based on etiology of bladder dysfunction. In patients with idiopathic DO or interstitial cystitis, the goals of treatment are to provide symptomatic relief, while avoiding negative side effects such as straining to void and urinary retention. However, in addition to improving QOL, the primary goals of BTX bladder injection in patients with neurogenic DO are to improve urodynamic storage parameters and minimize the risk of renal impairment. For these reasons, it is imperative that all NDO patients being evaluated for BTX injection undergo pretreatment urodynamics assessment. Because BTX chemical denervation is reversible over time, the main disadvantage of BTX injection is the need to undergo repeat injections. Fortunately, there is now evidence to suggest that repeat treatment is equally efficacious even after several injections.
Dosage, concentration, and injection technique
Published investigations to date have utilized varying doses, volumes, and injection target sites. These variations make systematic comparative assessment of the safety and efficacy of BTX difficult. A recent systematic literature review of BTX injection protocol characteristics reported that typically 300 U BTX-A (range 100–400 U) was injected in 30 sites (range 15–40) of 10 U/mL (range 6.7–25 U/mL) in the detrusor, with most investigators preferring to spare the trigone. Injection is typically performed under cystoscopic guidance (flexible or rigid), and can be performed under several types of anesthesia (none, local, spinal, or general).
Injection doses ranging from 100 to 400 U (Botox), and 500 to 1000 SU (Dysport) have been reported in published studies to date. The 300-U dose is the most consistently used across series, although isolated series have reported 100, 200, and 400 U. Although few studies have specifically investigated variable dosing, similar improvement has been reported regardless of total dose with respect to both subjective and objective outcomes. However, determining which dose offers maximum clinical effect with the least risk has not yet been determined. When an initial evaluation of BTX-A injection using 200 U and 300 U in 31 patients with NDO revealed that 2 patients receiving 200 U failed to show clinical improvement, Schurch and colleagues concluded that 300 U was the optimal treatment dose. However, prospective comparison of 200 U and 300 U in 59 patients with NDO showed significant subjective and objective improvement in all patients, with no differences between the 2 study arms. A recent meta-analysis of 8 randomized controlled OAB trials including BTX in at least one treatment arm reported that while low doses of BTX (100–150 U) appeared to have beneficial effects, higher doses (300 U) may be more effective. The investigators concluded that the optimal dose of BTX for efficacy and safety can not be determined until more definitive clinical trial data are available. A majority of investigations to date have utilized injection volumes ranging from 0.1 to 0.5 mL (6.7–25 U/mL)/injection site. Based on animal models, it has been proposed that larger dilution volumes will result in greater suburothelial diffusion with an increased treatment effect. However, this has not been demonstrated clinically, and there are concerns that larger volumes increase the potential for serosal extravasation and increased patient discomfort.
Although various techniques have been described, BTX injection is commonly performed under intravenous sedation or local anesthesia following administration of prophylactic antibiotics. In the operating room setting with the patient in the lithotomy position, a 21F rigid cystoscope and collagen injection needle are used to create a submucosal bleb under direct vision. If a flexible cystoscope is used, a longer needle and/or sheath for stabilization may be necessary. With increasing experience, the injection technique has been uneventfully performed in the office setting, using a flexible cystoscope under local anesthesia. This approach may facilitate cost reduction and avoidance of anesthetic risks.
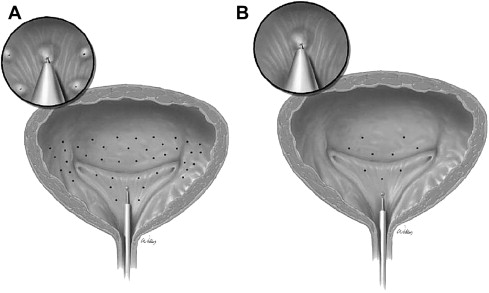
With endoscopic guidance, toxin is injected via 15 to 40 evenly distributed intramural injection sites including the bladder base and posterolateral walls ( Fig. 2 ). As a general principle, the bladder dome is excluded because of concerns regarding intraperitoneal perforation and bowel injury, and the trigone is spared to avoid inducing vesicoureteral reflux. In cases of DO arising from nonneurogenic cause and/or in patients with isolated pain/sensory components, recent protocols have included trigonal injection, with good results.
Treatment outcomes
A recent systematic literature review identified 18 studies investigating the use of BTX in patients with NDO. In this aggregate population of 698 patients, 83% had NDO with urinary incontinence refractory to high doses of anticholinergic therapy. Based on the aggregate outcomes, the investigators concluded that BTX detrusor injections provide a clinically significant benefit in adults with NDO and incontinence/OAB refractory to antimuscarinics. However, of the studies included in the systematic review, only 3 retrospective studies examined clinical series larger than 75 patients (all retrospective), and only 3 other studies were prospectively randomized with a control arm. The majority consisted of small open-labeled studies of less than 50 patients ( Table 1 ).
| Authors | Study Design (N) | BTX-A Preparation Dose (U) | No. of Injections | Incontinence Episodes/24 h | Pdet max (cm H 2 O) | MCC (ml) | Compliance (mL/cm H 2 O) | Mean Follow-Up (wk) | Adverse Events (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mean change from baseline (%) | |||||||||
| Retrospective Studies | |||||||||
| Reitz et al | MI (231) | Botox 300 | 30 | 73% continent 27% some improvement | −31 (51) d | 148 (35) d | 40 (125) d at 6 wk 19 (59) at 36 wk | 36 | N/A |
| Del Popolo et al | SI (199) | Dysport 500 (23%), 750 (53%), 1000 (24%) | 20 | Decreased IEs at 4 wk d Decreased pads/condoms at 4 wk d | N/A | 182 (80) c | N/A | 48 | Hyposthenia (2.5) |
| Stoehrer et al | SI (216) | Botox 300 Dysport 750 | Botox 30 Dysport 25 | >80% continent with CIC | −31.1 (56) d | 98 (33) d | 17 (73) at 6 wk d 8.6 (37) at 24 wk | 24 | Weakness (1) Dysphagia (0.5) Dysarthria (0.5) |
| Prospective Studies | |||||||||
| Schurch et al | RPC (59) | Botox 200, 300 | 30 | N/A | 24 | UTI (26.3) | |||
| Placebo (21) | −0.1 (3) | 1.4 (16) | 41.6 (16) | Injection site pain (5) UTI (14) | |||||
| BTX 200 U (19) | −1.1 (58) a | −38.7(67) a,e | 174.2 a (67) | UTI (32) | |||||
| BTX 300 U (19) | −0.9 a,e (32) | −35.5 a,e (32) | 92.9 a,e (32) | Injection site pain (11) UTI (21) | |||||
| Giannantoni et al | RACC (75) | Botox 300 | 30 | N/A | 112 | ||||
| RTX (35) | −2.8 (57) c | −8.6 (10) | 93.4 (40) b | ||||||
| BTX (40) | −4.0 c,e (77) | −32.9 (44) b | 134.6 (54) b,e | ||||||
| Ehren et al | RPC (31) | Dysport 500 | 25 | N/A | 26 | ||||
| Placebo (14) | N/A | −12 (21) | 10 (4) | 0 | |||||
| BTX (17) | −52 (77) c,e | 180 (62) c,e | Hematuria (6) | ||||||
In a large multi-institutional retrospective series, Reitz and colleagues reported significant increases in mean cystometric bladder capacity ( P <.0001), mean reflex volume ( P <.01), and decreased mean voiding pressures ( P <.0001) in 231 patients with NDO. Patients were treated with 300 U BTX-A in 30 trigone-sparing injection sites; and with 36 week follow-up, patients reported considerably reduced anticholinergic drug dose requirements and high subjective satisfaction rates, with no injection related complications or side effects. Stoehrer and colleagues reported the results of BTX-A detrusor injections in 216 patients with NDO, comparing maximal detrusor pressure, detrusor compliance, reflex volume, cystometric capacity, as well as use of anticholinergic agents and patient satisfaction at 6 weeks and 6 months after treatment. Using either 300 U of Botox or 750 U of Dysport, the investigators reported a significant improvement in all urodynamic parameters as well as incontinence rates and decreased anticholinergic use, but no significant differences were noted when comparing the 2 BTX-A preparations. Recently, Del Popolo and colleagues reported their findings using 1000 U, 750 U, and 500 U of BTX-A (Dysport) in 199 patients with spinal cord lesions and refractory NDO. Following urodynamic evaluation at baseline, then 3, 6, and 12 months post injection; significant improvements were noted in maximum bladder capacity, reflex volume, bladder compliance, and pad usage ( P <.001). Of note, there were no significant differences in therapeutic effect or duration of effect when comparing the differing dosing regimens.
In a double-blinded, randomized, placebo-controlled, 3-arm study comparing 200 U BTX-A, 300 U BTX-A, and placebo in 59 patients with NDO (90% SCI, 10% MS) requiring clean intermittent catheterization (CIC), Schurch and colleagues investigated frequency of urinary incontinence, maximum cystometric capacity (MCC), reflex detrusor volume, and maximum detrusor pressure via patient voiding diary and urodynamic assessment at 24 weeks post injection. The investigators reported significant improvement in QOL (Incontinence Quality of Life questionnaire), posttreatment decreases in incontinence episodes (IE), and improvement in urodynamic bladder function in both BTX treatment groups ( P <.05) from the first evaluation at week 2 to the end of the 24-week study when compared with baseline. The reported side effects with BTX use were minimal and symptom improvement was similar in the 200 U and 300 U dosing regimen groups, with no significant improvement noted in the placebo arm. Giannantoni and colleagues randomized 75 patients with SCI and refractory NDO to a 2-arm controlled trial comparing intravesical instillation of resiniferatoxin (RTX) dissolved in normal saline (N = 40) and 30 trigone-sparing bladder injections of 300 U BTX-A diluted in 30 mL normal saline (N = 35). Clinical assessment and urodynamics were performed at baseline, then 6, 12, and 24 months post treatment. The investigators reported significant reductions in mean catheterization rate and IE, as well as significant increases in mean first involuntary detrusor contraction and mean maximum bladder capacity at all time points in both treatment groups. Although side effects were not reported with either treatment, the investigators did note a significant benefit with BTX compared with RTX regarding number of IE per 24 hours and maximum detrusor pressure. In the most recent prospective study to date, Ehren and colleagues randomized 31 patients with NDO to a single treatment of 500 U BTX-A (Dysport) versus placebo using urodynamic parameters (6, 12, and 26 weeks), QOL, IE, and intake of rescue tolterodine as outcome measures. A significantly lower intake of tolterodine ( P = .003), increased cystometric capacity at 6 ( P <.001) and 12 ( P = .026) weeks, decreased maximum detrusor pressure ( P <.01), and decreased IE ( P <.01) were found in the BTX treatment arm. The consensus from these reports suggests that BTX achieves measurable urodynamic and subjective improvement in NDO patients with minimal negative sequelae. However, repeat injections are necessary to achieve a sustained response. Although contemporary series differ with regard to study methodology and injection protocols, here the authors summarize and quantify the reported outcomes within the broad categories of subjective patient-reported outcome measures and objective urodynamic parameters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






