Fig. 9.1
Structure of the CYT006-AngQb vaccine. The modified angiotensin II peptide is composed of the amino acid sequence of Ang Iotensin1–8 octapeptide (Angiotensin II) fused at its N-terminus to a spacer sequence containing a cysteine to permit directional conjugation to the Qb virus-like particle (VLP)
VLPs have supramolecular structures with rods or icosahedrons with diameters in the range of 25–100 nm. They are composed of multiple copies of one or more recombinant, expressed, viral, structural proteins which spontaneously assemble into particles. In addition to being virus-like in structure, they are often antigenically indistinguishable from the virus from which they were derived [16].
VLPs stimulate a strong B-cell response against self-antigens and this new technology helps overcome the self-tolerance limitations of immunization against angiotensin II [17, 18].
Tissot et al. did a multi-center, double-blind, randomized, placebo-controlled phase II trial. They randomized 72 patients with mild-to-moderate hypertension to receive subcutaneous injections of either 100 μg CYT006-AngQb, 300 μg CYT006-AngQb, or placebo, at weeks 0, 4, and 12 [19]. Twenty-four-hour ambulatory blood pressures were recorded before treatment and at week 14 . After a single injection, all patients receiving the vaccine responded with high anti-angiotensin II IgG titers. The antibody response was boosted after the second injection, and reached peak levels of response about 2 weeks after the third injection. The anti-angiotensin II IgG response was dose dependent, higher titers in patients randomized to 300 mcg than those randomized to 100-mcg dose. The half-life after the third injection was only 17 weeks. In the 300-mcg group, the ambulatory blood pressures fell 9.0/4.0 mmHg from baseline, with very dramatic decreases in early-morning blood pressures (25.0/13.0 mmHg).
The investigators could not document any change in the concentration of C1, C3, or factor C3a, which suggests that there was little to no immune complex deposition. The plasma renin levels increased in the vaccine group, likely due to a reduction in blood pressure. This trial is the first to show that vaccination against a vasoactive endogenous substance can reduce blood pressure in human beings .
Further work by the same team investigated more frequent dosing (at weeks 0, 2, 4, 6, and 10). This modification showed a fivefold increase in antibody titer but only − 2.3/− 0.4 mmHg improvement in blood pressure. Antibody affinities for angiotensin II were significantly lower in the second study than in the first (P < 0.001). The authors concluded that both the quantity and the quality of the antibody is important for blood pressure reduction. Future studies on CYT006-AngQb are on hold due to financial reasons .
Angiotensin II Receptor Type 1 Vaccine
The newest vaccine approach does not target renin, angiotensin I or angiotensin II, rather it creates antibodies to block angiotensin II receptor type 1 (ATR; Fig. 9.2) .
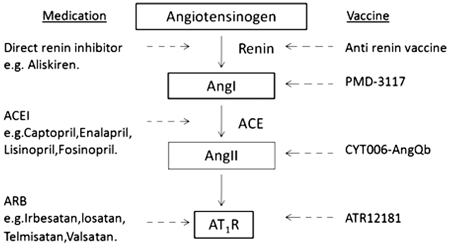
Fig. 9.2
Schematic representation of the classic renin–angiotensin system with oral medication and vaccines blocking at their specific targets. The inhibitory actions are shown in dashed lines with arrows. ACE angiotensin converting enzyme, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, AT 1 R Ang II-type 1 receptors
Zhu et al. immunized spontaneous hypertensive rat with a peptide-based vaccine made of a seven-amino-acid sequence (AFHYESR) from the second extracellular loop of rat AT−1 A receptor (ATR12181; [20, 21]). The carrier protein is a TT complex in combination with Freund’s adjuvant. The vaccine induced anti-ATR12181 antibodies and a 17-mmHg reduction in systolic blood pressure. They also noted decreased cardiac hypertrophy and decreased kidney injuries. No signs of autoimmune disease were found after sacrificing the rats.
The same group changed the adjuvant to VLP, which have a better safety profile than Freund’s adjuvant [22]. The vaccine significantly decreased the blood pressure of angiotensin II-induced hypertensive mice up to 35 mmHg and that of spontaneously hypertensive rats up to 19 mmHg and prevented remodeling of hypertensive-vulnerable target organs. The half-life of the antibody was 14.4 days. The antibody specifically bound to angiotensin II receptor type 1 and inhibited angiotensin II- induced calcium-dependent signal transduction events, including protein kinase C-α translocation, extracellular signal-regulated kinase 1/2 phosphorylation (72 % decrease; P = 0.013). They also saw a 68 % decrease in intracellular Calcium (P = 0.017). The antibody did not inhibit angiotensin II binding to the receptor but rather diminished the pressure response and signal transduction initiated by angiotensin II .
Road Block and Future Direction
A vaccine for hypertension has been investigated for over 100 years. Though an effective therapy has not emerged, the journey has resulted in significant scientific breakthroughs. Summary of the clinical trial of hypertension vaccine is given in Table 9.1. The earliest efforts used renin vaccines. This was a proof of concept. By transferring renin across species, renin could be made immunogenic and the anti-renin antibodies effectively lowered blood pressure in animals. Unfortunately, the effectiveness came with devastating autoimmune consequences forcing the abandonment of this target molecule. The autoimmune complications were at least partly due to the large size of the antigen [23]. Renin is a 406-amino acid peptide while angiotensin I , angiotensin II, and the angiotensin receptor target-region are 10, 8, and 7 amino acid peptides respectively. The smaller molecular target decrease the likelihood of simultaneous binding of two antibodies to a single antigen resulting in less cross-linking and decreased immune-complex formation.
Table 9.1
Summary of hypertension vaccines tested in human trials
Title
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|



