Bladder Cancer
Steve Williams
Merce Jorda
Murugesan Manoharan
Mark Soloway
Introduction
Bladder cancer ranks as the fourth leading cause of cancer-related death in men and tenth leading cause in women in the United States (Parkin et al., 2002). A delay in the diagnosis and treatment of bladder cancer results in a poorer outcome (Sanchez-Ortiz et al., 2003). An interval of longer than 3 months from the time of diagnosis of muscle invasion to cystectomy correlates with a higher pathological stage and decreased survival (Chang et al., 2003; May et al., 2004). Early recognition of symptoms of bladder cancer by primary care physicians with prompt referral and evaluation and treatment by a urologist is necessary for a successful result.
Diagnosis
Early symptom recognition is the key to a better prognosis. The most common symptom of bladder cancer is painless haematuria, which occurs in 85% of patients. Nearly all patients with bladder cancer have at least microhaematuria if sufficient urine samples are tested (Messing and Vaillancourt, 1990). The degree of haematuria does not correlate with the extent of disease. Haematuria may be measured by indirect examination of the urinary sediment by the dipstick method, by direct examination of the centrifuged sediment (sediment count) or by determination of the number of red blood cells per millilitre of urine excreted (chamber count).
The most common method of screening patients for haematuria is haemoglobin dipstick. A sample is recorded as positive when any haemoglobin is present. The haemoglobin dipstick method is easy to use, inexpensive and does not require the presence of intact red blood cells. Dipstick testing for haematuria has a limited specificity (between 65% and 99%); therefore, an initial positive result by this method should be confirmed by microscopic evaluation of the urinary sediment (Messing et al., 1987; Woolhandler et al., 1989; Sutton, 1990). Because haematuria is intermittent in nature, repeat testing increases the sensitivity of the haemoglobin dipstick test.
Microscopic haematuria has been defined as three or more red blood cells per high power field on microscopic evaluation of urinary sediment from two of three properly collected urinalysis specimens (Grossfield et al., 2001). Patients with risk factors for significant disease (Table 6.1) should undergo a thorough evaluation for any degree of haematuria. The prevalence of asymptomatic microscopic haematuria in the general population ranges from 0.19% to 21% (Golin and Howard, 1980; Mohr et al., 1986; Sultana et al., 1996; Khadra et al., 2000). Mohr et al. (1986) reported that asymptomatic microhaematuria occurred in 13% of the general population and only 0.4% had urothelial neoplasia. The incidence of malignancy in patients over 50 years is higher, however. Sultana et al. (1996) found that 5% of patients older than 50 years with asymptomatic haematuria had an underlying malignancy. The incidence increased to 10% if there were associated symptoms.
Table 6.1 Risk factors for significant disease in patients with microscopic haematuria | ||||||
|---|---|---|---|---|---|---|
|
Screening for haematuria in the general population is not recommended because the positive predictive value (PPV) is too low. It may, however, be reasonable for a high-risk population, such as those exposed to bladder carcinogens such as cigarette smoke, aniline dyes or aromatic amines (Kirkali et al., 2005). The risk of bladder cancer among these high-risk patients is two- to eightfold higher than in normal population after correcting for age, gender and race.
Symptoms that may be caused by bladder cancer include bladder irritability, i.e. urinary frequency, urgency and dysuria. This is a relatively infrequent but important presentation of bladder cancer, and is usually associated with carcinoma in situ (CIS) or high-grade bladder cancer (Utz and Farrow, 1984). Late symptoms and signs of bladder cancer include flank pain (due to ureteral obstruction), lower extremity oedema, a pelvic mass, weight loss or bone pain.
Urinary cytology
Urinary cytopathology (UC) or cytology is the microscopic examination of voided urine or a bladder wash specimen for malignant urothelial cells. UC is used primarily to monitor patients with a history of bladder cancer for the detection of recurrences. UC is highly tumour-specific (>90-95% specificity in most studies), but the sensitivity reported in various studies ranges from 11% to 76% (average 40%) (Bastacky et al. 1999). The overall low sensitivity of cytology to detect bladder cancer is almost exclusively due to its inability to detect low-grade bladder tumour (sensitivity 15-20%; Bastacky et al., 1999). Owing to cost and low yield, UC is therefore not particularly suited for screening the general population. Examination of a voided urine or bladder barbotage specimen for exfoliated cancer cells is more accurate when a high-grade cancer is present (Murphy, 2000). It is especially useful for patients treated with topical agents, as the effects of therapy may confound cystoscopic examination. Cytology may identify the presence, although not the location, of a high-grade urothelial carcinoma located in the urethra, prostatic ducts, or in the upper tracts.
UC can assist the urologist in the timing of cystoscopy during patient monitoring. Patients being observed after the diagnosis of a papillary urothelial neoplasm of low malignant potential (PUNLMP) or non-invasive low-grade carcinoma who have no recognizable tumour cells in their urinary specimens can undergo cystoscopy at longer intervals than patients with tumour cells in their UC (Soloway et al., 1990). UC interpretations can be of prognostic value. Patients treated with topical therapy who have high-grade cancer cells in their urine are more likely to require a cystectomy (Kirkali et al., 2005).
Terminology
Because the features used to classify urothelial neoplasms histologically may not be present in the cytological sample (namely, the stalk), Murphy (2000) has recommended the following terminology to communicate the cytopathological interpretation: (1) positive, consistent with low-grade neoplasm (6.1); (2) positive, consistent with high-grade neoplasm (6.2 and 6.3); (3) suspicious for high-grade neoplasm; (4) dysplastic cells, rule out low-grade neoplasm; (5) negative, neoplastic cells not identified; and (6) unsatisfactory, insufficient cells for interpretation. The histological correlates of these cytological diagnoses are noted in Table 6.2.
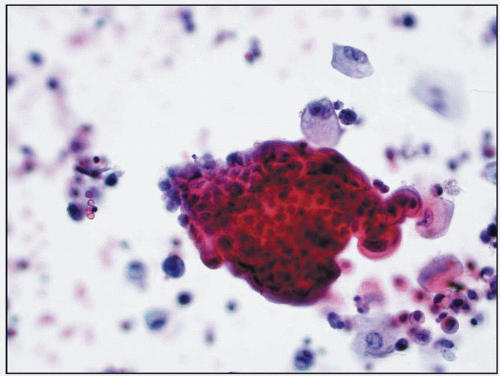 6.1 Clusters of cells from low-grade urothelial carcinoma. The background is clean and inflammatory cells are not present (urine cytology, Papanicolaou stain, x40). |
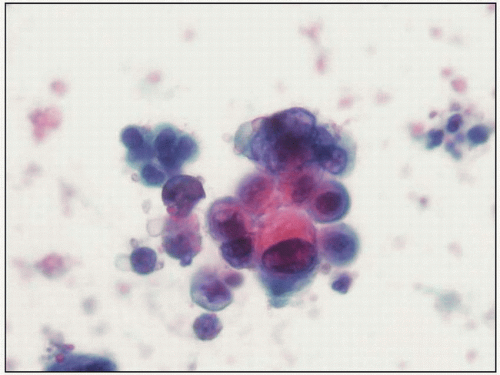 6.2 Clusters of cells from high-grade urothelial carcinoma. Here some of the cells are vacuolated and pleomorphic (urine cytology, Papanicolaou stain, x40). |
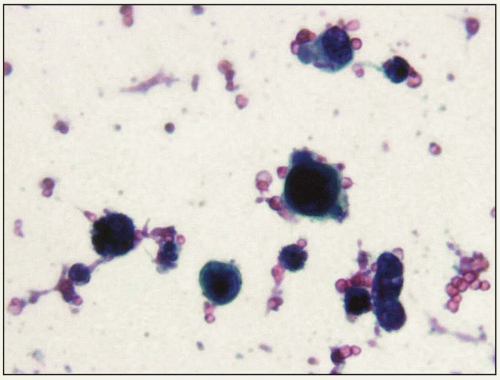 6.3 Isolated cells from high-grade urothelial carcinoma representing in situ urothelial carcinoma (urine cytology, Papanicolaou stain, x40). |
The term suspicious is used to describe cells that have features of a high-grade neoplasm, but there are too few for an unequivocal interpretation. Those who have studied asymptomatic, high-risk individuals have discovered that factors other than a developing neoplasm can cause cells in a urinary specimen to appear neoplastic (Farrow and Utz, 1982; Crosby et al., 1991). It is also best to be cautious with the interpretation of a few malignant-appearing cells in specimens from untreated individuals because high-grade urothelial neoplasms in such cases should shed numerous cells into the urine. Dysplastic cells warrant attention, but in many cases, they are not considered diagnostic.
Diagnostic yield of urinary cytopathology
The value of UC in the detection of urothelial neoplasms depends on a variety of factors. One variable affecting the sensitivity of UC is the type of specimen from which interpretations are made. Voided specimens may occasionally be hypocellular and degenerated. They may also contain significant amounts of skin and vaginal contamination, particularly in female patients. Catheterized urine and bladder washes typically contain more cells and less contamination and, as such, have a 20% higher sensitivity (Gregoire et al., 1997; Glas et al., 2003). Properly performed, a bladder washing should include the residual urine collected when the catheter or other instrument is introduced plus a vigorous lavage done after a cystoscopy. Neither random urine specimens nor bladder washings contain tumour cells in every specimen. Malik and Murphy (1999) found that 23% of washings from patients with biopsy-proven, high-grade urothelial carcinomas in their bladders contained no tumour cells. This forms the basis for the recommendation by some authorities that UC specimens should be obtained on three separate days (Badalament et al., 1987).
False-positive cytology results are uncommon. Some patients have positive urine cytology detected with no clinically demonstrable disease. These patients are more likely to have a small urothelial neoplasm that was missed at cystoscopy. There are several reports of patients with positive cytology with normal findings at cystoscopy and upper tract evaluation that required months for histological correlation (Farrow et al., 1977; Murphy et al., 1984). Most recurrences are evident clinically within 3 years of a detectable positive cytology, and therefore close monitoring of the patient (every 3-6 months) during this period is recommended (Schwalb et al., 1994; Dalbagni et al., 1999). Cytological specimens obtained immediately in the post-operative or post-intravesical therapy period may also result in a false-positive reading due to cautery artefacts, inflammation or degradation of cells from instrumentation (Muller et al., 1985).
Other factors that may affect the diagnostic yield include: (1) definition of a cytohistological correlation (whether an immediate or a delayed histological correlation); (2) grade of tumour; (3) whether the correlation is cystoscopic only; (4) number of cytopathologists involved and their interaction with each other as well as their involvement in the data analysis; (5) types of cases (primary neoplasms, persistent or recurrent neoplasms); (6) case mix (the percentage of neoplasms versus negative results will affect the specificity and the predictive value); (7) presence or absence of topical therapy; and (8) histopathological classification used for correlation.
In summary, UC is best applied for the follow-up of patients following a diagnosis of a high-grade urothelial neoplasm. Given adequate sampling and ≥3 specimens, up to 90% of high-grade urothelial carcinomas can be detected by cytology, and the PPV is >90%. UC is less valuable for the detection of low-grade urothelial neoplasm and can be used for monitoring patients who have low-grade non-invasive urothelial neoplasms to detect any high-grade tumours that might develop. Given an adequate sample, the PPV for an
interpretation of high-grade neoplasm in a UC is so high that it can almost never be considered falsely positive. In contrast, the PPV for interpretations of low-grade neoplasm and ‘dysplastic cells, rule out low-grade neoplasm’ is approximately 60%, high enough to warrant a cystoscopy but not selected site biopsies.
interpretation of high-grade neoplasm in a UC is so high that it can almost never be considered falsely positive. In contrast, the PPV for interpretations of low-grade neoplasm and ‘dysplastic cells, rule out low-grade neoplasm’ is approximately 60%, high enough to warrant a cystoscopy but not selected site biopsies.
The cellular features of urothelial neoplasms
The cellular features of urothelial neoplasms have been described and illustrated in numerous publications (Malik and Murphy, 1999; Murphy, 2000). Because the features used for histological distinctions may not be present in the disaggregated cells of cytological samples and because it is not always possible to distinguish glandular neoplasms from urothelial tumours, the most accurate approach to classification in this area would seem to be separation on the basis of degree of cytological anaplasia (Tables 6.2 and 6.3).
Table 6.2 Histological correlates to cytologic diagnoses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Table 6.3 Cytological features evaluated for the diagnosis of malignancy | ||||||
|---|---|---|---|---|---|---|
|
High-grade neoplastic cells (6.2) may be numerous or sparse, depending on the type of specimen, the approach to collection, and the previous application of topical therapy. The key diagnostic changes are nuclear pleomorphism and coarsely granular, irregularly dispersed chromatin. Large nucleoli may appear in high-grade neoplastic cells but are rarely numerous and not essential for interpretation. Occasionally, cells may be small, with degenerated nuclei lacking chromatin detail, but the increased nuclear-cytoplasmic ratios and peculiar nuclear shape of these cells tend to reveal their nature. Importantly, nearly all high-grade neoplastic cells contain all of the diagnostic features listed in Table 6.3.
The cells of low-grade urothelial tumours lack many features associated with malignancy, such as nuclear pleomorphism, coarsely clumped chromatin and large nucleoli (6.1). In tissue specimens, low-grade urothelial neoplasms are recognized as neoplastic primarily because their cells are arranged on delicate fibrovascular stalks. Similar cells in a flat urothelium cannot be recognized as neoplastic in histological sections and are often termed dysplasia. In other words, it is not the cells, but the arrangement of the urothelium, that allows the pathologist to diagnose a low-grade urothelial neoplasm.
Low-grade urothelial neoplasms are composed of cells lacking many features of malignancy. They can be construed as lesions composed of dysplastic cells on delicate fibrovascular stalks. It is the stalk, rather than the cells, that allows histological classification of these tumours as neoplasms. When disaggregated in urinary samples, there is little difference between the cells on stalks and similar cells that might occupy flat areas of urothelium. Therefore, low-grade and dysplastic cells will be described together.
Low-grade/dysplastic cells are often numerous in urinary specimens. The abnormally high number of cells is often the most important clue to the presence of a low-grade carcinoma or PUNLMP and should be reported as ‘numerous cells, a very low-grade neoplasm cannot be excluded’, even if the cells themselves appear relatively normal. Neoplastic cells tend to be loosely clustered. They have markedly eccentric nuclei and increased nuclear-cytoplasm ratios. The nuclei are irregular, a feature usually manifested by a single notch, crease or shallow depression. The nuclear chromatin is more granular than normal but evenly dispersed. Large nucleoli are not a feature of these cells but are typical of the reactive or regenerative elements from which they must sometimes be distinguished. Importantly, all of the features listed in Table 6.2 are not necessarily present in every cell.
Limitations in the use and interpretation of urinary specimens
Consultations based on urinary specimens have been extremely beneficial in certain clinical situations, primarily
to identify high-grade neoplastic cells in the bladders of patients being monitored for persistent or recurrent disease. The use of UC has limitations in the following areas: (1) screening the asymptomatic population; (2) detection of renal parenchymal carcinoma; (3) detection of prostatic adenocarcinoma; (4) localization of a neoplasm; (5) detection of a non-aggressive neoplasm; (6) detection of adenocarcinomas and squamous cell carcinoma of the urinary bladder; (7) detection of a non-urothelial neoplasm; (8) detection of neoplasms with little or no surface components.
to identify high-grade neoplastic cells in the bladders of patients being monitored for persistent or recurrent disease. The use of UC has limitations in the following areas: (1) screening the asymptomatic population; (2) detection of renal parenchymal carcinoma; (3) detection of prostatic adenocarcinoma; (4) localization of a neoplasm; (5) detection of a non-aggressive neoplasm; (6) detection of adenocarcinomas and squamous cell carcinoma of the urinary bladder; (7) detection of a non-urothelial neoplasm; (8) detection of neoplasms with little or no surface components.
Summary
The cytopathological assessment of urinary specimens is a valuable means to detect tumour cells in patients suspected of harbouring a bladder cancer. Despite its limitations, this approach is currently the single most efficacious way to monitor patients for clinically important disease after cystoscopy.
Urine markers
Although the ideal use of a urine-based marker for the diagnosis of bladder cancer has not been determined, several tests have been approved for use by the US Food and Drug Administration (FDA). These include the bladder tumour antigen (BTA), the BTA stat (Polymedco Inc., Redmond,WA, USA), the nuclear matrix protein 22 (NMP22) (Matritech, Newton, MA, USA), and UroVysion (Abbott Molecular/ Abbott Laboratories Inc., Des Plaines, IL, USA).
BTA stat
The BTA stat is a point-of-care test approved by the FDA as an adjunct to cystoscopy for monitoring bladder cancer recurrence. This test detects the presence of human complement factor H-related protein in the urine. In a side-by-side comparison of tumour markers, BTA stat was found to have a much higher sensitivity for high-grade tumours (74%) than for low-grade tumours (25%). Its specificity was 77% (Lokeshwar et al., 2002).
NMP22
The NMP22 is a point-of-care test and detects elevated nuclear mitotic apparatus protein (6.4). The NMP22 test is designed to measure NMP22 levels in a patient’s urine by a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) that uses two monoclonal antibodies (6.4).
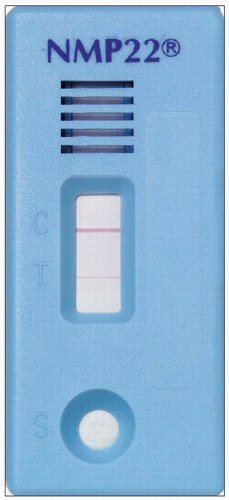 6.4 NMP22 positive test. Note positive reading indicated by a red line in both the control (C) and test (T) windows. |
The sensitivity and specificity of NMP22 has been evaluated in many studies. A study evaluating the detection of bladder cancer with this test and cytology found the NMP22 to have a specificity of 86% (Gutierrez Banos et al., 2001). In studies that have compared the sensitivity of NMP22 according to tumour grade, the test has lower sensitivity to detect low-grade tumours (Gutierrez Banos et al., 2001; Ponsky et al., 2001). However, in most studies, the sensitivity of the NMP22 test for detecting intermediate- and high-grade tumours varies between 50% and 70%, and 70% and 100% respectively.
UroVysion test
The UroVysion test is comprised of a multicoloured fluorescence in situ hybridization probe set approved by the FDA for the detection of bladder cancer. It involves the staining of exfoliated cells in urine with four denatured fluorescent centrometric chromosome enumeration probes. These detect chromosomes 3, 7 and 17 and a locus-specific probe for 9p21. The cells are observed under a fluorescence microscope. The set criteria for detecting bladder cancer by the UroVysion test are ≥5 cells with a gain of ≥2 chromosomes, ≥10 cells with a gain of one chromosome, or ≥20% of cells
with a loss of 9p21 locus. In various studies, the sensitivity of the UroVysion test is between 69% and 87% (Lokeshwar et al., 2004, 2005). The test has excellent sensitivity to detect CIS and high-grade tumours (range 83-100%). It is useful as an adjunct to cytology because it maintains the specificity but increases the sensitivity (45.8% vs 72.22%) (Halling et al., 2000; Sokolova et al., 2000).
with a loss of 9p21 locus. In various studies, the sensitivity of the UroVysion test is between 69% and 87% (Lokeshwar et al., 2004, 2005). The test has excellent sensitivity to detect CIS and high-grade tumours (range 83-100%). It is useful as an adjunct to cytology because it maintains the specificity but increases the sensitivity (45.8% vs 72.22%) (Halling et al., 2000; Sokolova et al., 2000).
Immunocyt test
The Immunocyt test is based on the visualization of tumour-associated antigens in urothelial carcinoma using fluorescein-labelled monoclonal antibodies. The monoclonal antibodies M344 and LDQ10 are directed against sulphated mucin glycoproteins and a Texas red-linked monoclonal antibody 19A211. Specific technical requirements for this assay are a high-quality fluorescence microscope and a centrifuge. In several studies, the sensitivity of Immunocyt varies between 38% and 100%, and the specificity ranges between 75% and 90% (Pfister et al., 2003; Hautmann et al., 2004). The test appears to have a similar sensitivity in detecting low-grade, low-stage tumours (i.e. 18-100%) and high-grade, high-stage tumours (77-100%). Immunocyt, with an average sensitivity and specificity of approximately 80%, is superior to conventional urine cytology and is clearly among the most promising diagnostic markers for bladder cancer.
These and other urine-based markers under development can provide advantages over cytology. An ideal protocol for the adjunctive use of ≥1 of these tests in combination with cystoscopy and cytology will require further studies and discussion among opinion leaders.
Imaging of bladder cancer at initial diagnosis
Evaluation of the upper urothelial tract
All patients with haematuria must have their upper tracts evaluated. Excretory urography (intravenous urography, IVU




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








