Fig. 13.1
(a) Position of the segment III bile duct and the use of intraoperative ultrasound to aid location. (b) A 2 cm bilioenteric anastomosis is constructed using an isolated Roux loop. (c) The finished bilioenteric anastomosis after completion of the anterior row of sutures (Reprinted with permission from Dennison and Maddern [5])
For cholestasis to be relieved, a minimum of 30 % of the liver parenchyma, or two liver segments, should be drained [7]. It has long been recognised that unilateral hepatic drainage can successfully palliate hilar cholangiocarcinomas even when communication between the left and right ductal systems is prevented by tumour growth [8]. Relative contraindications for a segment III cholangiojejunostomy include infection in an obstructed right ductal system (commonly precipitated by previous attempts at stenting), atrophy of the left lobe arising secondary to vascular involvement, extensive metastases in the left lobe and tumour involvement of the secondary branches of the left ductal system [6, 7, 9].
Most studies reporting the outcomes for segment III cholangiojejunostomies include cohorts of patients with hilar cholangiocarcinomas and gall bladder cancers [6, 9–13]. Focusing only on hilar cholangiocarcinomas, segment III cholangiojejunostomies are associated with a morbidity rate of 17–55 % and a perioperative mortality rate of 0–33 % [6, 7, 10]. Mean survival following the procedure is 5–9 months, with a maximum survival of 19 months (Table 13.1) [7, 10].
Table 13.1
Outcomes following segment III cholangiojejunostomy for hilar cholangiocarcinoma
Authors | Number of patients with hilar cholangiocarcinomas | Morbidity | Number of biliary leaks | Perioperative mortality | Survival, months |
|---|---|---|---|---|---|
Blumgart et al. [14] | 28 | NR | NR | 6 (21 %) | NR |
Traynor et al. [7] | 48 | 8 (17 %) | 4 (8 %) | 3 (6 %) | Mean, 9.2 |
Maximum, 19 | |||||
Jarnagin et al. [6] | 20 | 11 (55 %) | 6 (30 %) | 0 | Median, 18.5 |
Launois et al. [10] | 12 | NR | NR | 4 (33 %) | Mean, 5 |
Maximum, 11 |
Factors associated with significantly reduced survival following a segment III cholangiojejunostomy for cholangiocarcinoma include development of an anastomotic leak and stage IV disease [6]. Jarnagin et al. describe a subgroup of 20 patients who underwent segment III bypasses for hilar cholangiocarcinoma. Of these 20 patients, seven (35 %) were readmitted with biliary sepsis, and three (15 %) required further biliary intervention. The patency rate for segment III bypass at 1 year was 80 %.
Traynor et al. report quality of life following segment III bypasses for hilar cholangiocarcinoma in the form of a “comfort index”, the duration of symptom-free survival expressed as a percentage of total survival [7]. They describe a comfort index of 91 % with a mean survival of 9.2 months. This compares with a comfort index after stenting in the region of 50 % [11]. Of the 48 patients undergoing a segment III bypass described by Traynor et al., bilirubin levels normalised in 35 patients (73 %) fell to 50 % of their preoperative value in 11 patients (23 %) but fell by less than 50 % in the remaining two patients [7].
13.1.1.2 Right-Sided Cholangiojejunostomy
For those patients in whom a segment III bypass is not feasible, a right-sided cholangiojejunostomy may be undertaken. Right-sided drainage requires identification of either the right anterior sectoral or segmental ducts, which is aided by intraoperative ultrasound following cholecystectomy (Fig. 13.2).
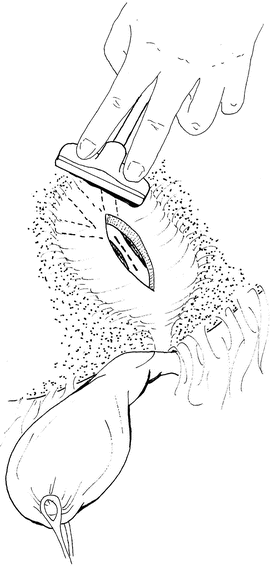

Fig. 13.2
Following a cholecystectomy, the segment V duct is located using intraoperative ultrasound. Depending on the position of the duct, a hepatotomy may be required to facilitate the anastomosis (Reprinted with permission from Dennison and Maddern [5])
Division of the liver parenchyma in the base of the gall bladder fossa exposes the anterior sectoral duct that is usually utilised for right-sided intrahepatic bypasses [15]. A side-to-side biliary-enteric anastomosis is constructed using a Roux-en-Y loop of jejunum.
Jarnagin et al. report outcome data from 14 patients who underwent a right sectoral bypass for hilar cholangiocarcinoma [6]. There was a significantly higher 30-day mortality in patients undergoing a right-sided bypass than in those undergoing a segment III bypass (21.4 vs. 0 %). In addition, the rate of late bypass failure was greater for those undergoing a right sectoral bypass, and this group also required a significantly greater number of postoperative biliary interventions (55 % right sectoral vs. 15 % segment III). These observations probably reflect the greater technical challenge associated with right-sided intrahepatic bypasses and emphasise the fact that a segment III cholangiojejunostomy is preferable.
13.1.2 Distal Cholangiocarcinoma
As for hilar cholangiocarcinomas, the majority of patients requiring palliative treatment for unresectable distal disease are managed with a biliary stent. Stents for distal biliary obstruction are easier to place than those for hilar obstruction and have a greater long-term patency rate [16]. A recent meta-analysis comparing stenting and surgical bypass for malignant distal biliary obstruction revealed no difference in therapeutic success rates between the procedures. A trend in 30-day mortality in favour of stenting was observed; however, the risk of recurrent biliary obstruction was significantly higher in this group [17]. These studies generally include patients with malignant distal biliary obstruction arising secondary to a number of causes, most frequently pancreatic cancer, rather than solely including patients with distal cholangiocarcinomas [18–22].
Although most patients with distal cholangiocarcinomas are managed palliatively with a biliary stent, the indications for palliative surgery are similar to those in hilar disease. Surgical bypass should be considered in patients with a distal cholangiocarcinoma in whom stenting fails or in those who are found to have unresectable disease at exploratory laparotomy. In addition, it should also be considered in occasional patients with a relatively long estimated survival [3]. A number of factors have been associated with longer survival in patients with malignant distal biliary obstruction, including the absence of metastatic disease and symptomatic gastric outlet obstruction, a favourable American Society of Anesthesiologists (ASA) score, the absence of pain and a normal C-reactive protein (CRP) and white cell count [21, 23, 24].
13.1.2.1 Hepaticojejunostomy/choledochojejunostomy
The preferred procedure to achieve surgical palliation for patients with distal cholangiocarcinoma depends on the exact site of the tumour but is either a hepaticojejunostomy or choledochojejunostomy. It has long been recognised that these bypasses yield superior results compared to those involving the gall bladder or duodenum [25]. Concomitant gastrojejunostomy as a prophylactic measure to avoid gastric outlet obstruction is now recommended by most authors [26]. Although only 5 % of patients with malignant distal biliary obstruction exhibit gastric outlet obstruction at diagnosis, it can develop in up to 20 % of patients as the disease progresses [27]. Several recent studies including patients with malignant distal biliary obstruction, most of whom underwent combined biliary and gastric bypasses, have shown perioperative mortality rates of 0–4 % and median survival ranges from 6.4 to 8.3 months [21–23]. In the largest study of 269 patients, 9 % required further surgery, which largely reflects the fact that 23 % of patients who did not receive a gastrojejunostomy subsequently developed gastric outlet obstruction [21].
Over the last 10 years, there have been increasing reports of laparoscopic bypass procedures. The development of laparoscopic stapling devices has allowed gastrojejunostomies and cholecystojejunostomies to be conducted with relative ease. However, a significant proportion of patients are not suitable for a cholecystojejunostomy due to tumour involvement at the junction of the cystic and hepatic ducts or previous cholecystectomy [28]. Laparoscopic hepaticojejunostomies and choledochojejunostomies have been described, but they are technically challenging and not widely practised. Operating time associated with these procedures is significant, and studies to date consist of small numbers of patients.
13.1.3 Gall Bladder Cancer
Unresectable gall bladder cancer is associated with a dismal survival of approximately 2–5 months and nonsurgical methods of palliation should therefore be undertaken in the vast majority of patients. If unresectable disease is discovered at exploratory laparotomy a segment III bypass may be considered to relieve jaundice. However, mortality associated with this bypass in patients with gall bladder cancer is as high as 17 %, and few would reap any long-term benefit [6]. This group of patients should therefore be managed with postoperative endoscopic or percutaneous biliary drainage in preference to a biliary-enteric bypass.
13.1.4 Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) is a progressive cholestatic liver disease of unknown aetiology that is associated with inflammatory fibrosis and strictures of the intra- and extrahepatic ducts. The prognosis of patients with PSC is poor, and the median survival from diagnosis to death or liver transplantation is only 9.6 years [29]. Dominant strictures of the extrahepatic bile ducts have been described in 10–20 % of patients with PSC and are one of the primary indications for intervention, in the form of palliative endoscopic, percutaneous or surgical techniques [30–33].
Dominant strictures of the common bile duct and common hepatic duct are defined as those reducing the diameter of the duct to ≤1.5 and ≤1.0 mm, respectively [34, 35]. Although usually asymptomatic in the early stages, progression can give rise to worsening jaundice, deteriorating liver function tests and cholangitis. As PSC predisposes to cholangiocarcinoma, making a distinction between benign and malignant strictures clinically can be difficult. At presentation approximately 25 % of dominant strictures are malignant [36], and brush cytology at endoscopic retrograde cholangiopancreatography (ERCP) may provide a diagnosis. A recent prospective study of brush cytology obtained at ERCP revealed the sensitivity for diagnosing cholangiocarcinoma to be 80 % [37]. In terms of radiology, positron emission tomography (PET) scanning is superior to conventional investigations (CT and MRI) in differentiating between PSC and cholangiocarcinoma [38]. If diagnostic doubt regarding the nature of a dominant stricture exists despite investigation, surgical excision is indicated.
Non-cirrhotic patients with PSC who are symptomatic with cholestasis from a dominant stricture can be treated with endoscopic, percutaneous or surgical interventions. Non-operative interventions have several advantages, including lower complication rates, avoidance of a general anaesthetic and the ability to repeatedly intervene without altering biliary anatomy or compromising the possibility of a future liver transplant. They should therefore be regarded as first-line procedures for the treatment of symptomatic dominant strictures in non-cirrhotic patients with PSC. Many surgeons now advocate orthotropic liver transplantation as the only definitive form of treatment for non-cirrhotic patients with a dominant stricture; however, excision of the extrahepatic biliary tree and reconstruction using a Roux loop have been described [39–41]. Undoubtedly, cirrhotic patients with PSC are best managed with a liver transplant as other surgical treatments are associated with high operative mortality rates and poor long-term survival [42].
13.1.4.1 Biliary Resection and Bypass
In most patients with PSC, the hepatic duct bifurcation is the region most severely affected [43]. As such, resection of the extrahepatic ducts along with the hepatic bifurcation and reconstruction with a hepaticojejunostomy has been described. A number of authors have also advocated the use of long-term bilateral transhepatic biliary stents with this approach, to reduce the risk of future strictures [39–41]. A recent study described outcome data for 77 patients who underwent resection of the extrahepatic ducts and hepatic bifurcation with hepaticojejunostomies and transhepatic biliary stents. The 30-day mortality for these patients was 3.9 % with a perioperative complication rate of 38.7 %, the most frequent complications being cholangitis (24 %) and a bile leak (9.1 %). Bilirubin levels fell significantly postoperatively, and at 3 years, 57 % of patients had no PSC-related admissions. Survival rates at 5 and 10 years were 76 and 53 %, respectively [39]. When transhepatic stents are inserted, these require multiple replacements in the long term. However, of more concern is the fact that surgery involving the biliary tree can increase the morbidity and mortality associated with subsequent liver transplant [44–46].
In summary, palliative excision of the biliary tree and reconstruction using a Roux loop for PSC have been described. However, this approach has declined in popularity due to improvements in endoscopic techniques, greater access to liver transplantation and concerns regarding the potential impact on future transplantation. Although still advocated by some authors [39], most patients can now be treated symptomatically with endoscopic techniques, and these can act as a bridge to transplantation when required.
13.2 Liver
Most liver resections should be undertaken with curative intent. In the palliative setting, they are undertaken with two main aims, either to prolong survival or for symptom control. Neuroendocrine tumours are one of the most frequent indications for palliative liver resections, often yielding benefits both in terms of survival and symptom control. The morbidity associated with a liver resection is of particular concern when this is undertaken with palliative intent and the overall prognosis associated with the tumour is an important consideration (Table 13.2).
Table 13.2
The prognosis of hepatic metastases and the site of the primary tumour
Bad prognosis associated with liver metastases | Intermediate prognosis associated with liver metastases | Better prognosis associated with liver metastases |
|---|---|---|
Pancreas | Sarcoma | Kidney |
Stomach | Gastrointestinal stromal tumours | Adrenal |
Cutaneous melanoma | Breast | Gynaecological/testicular tumours |
Palliative liver resections are far more appealing in those tumours that are associated with a better prognosis, for example, renal tumours. Although there is very strong evidence to support the role of cytoreductive hepatic surgery in neuroendocrine tumours, evidence for its role in prolonging survival in other tumour types is far more limited. Resection may effectively palliate symptoms associated with liver tumours in a select group of patients in whom all conservative approaches fail.
13.2.1 Neuroendocrine Tumours
Neuroendocrine tumours (NETs) are a diverse group of neoplasms that are characterised by their relatively slow rate of growth along with their propensity to produce and secrete hormones and other vasoactive substances. NETs are relatively uncommon tumours; however, evidence suggests the incidence is increasing and it is currently approximately five per 100,000 [47]. Around 85 % of NETs originate from the gastrointestinal tract, and the majority of patients have liver metastases at the time of diagnosis, which substantially reduces survival. Curative (R0) surgical resection is only possible in approximately 15 % of patients with neuroendocrine liver metastases (NELM); frequently, the extent and localisation of disease means that palliation is the only treatment option [48].
13.2.1.1 Liver Resection: Rationale
The main aims of palliative liver resections for NETs are to improve symptoms (and associated quality of life) and facilitate the effect of non-operative treatments. Palliative resections for NELM can also confer a survival benefit. Symptoms from NETs that resections may palliate relate to the mass effects of the tumour and those arising from hormones and peptides secreted by functional tumours (Table 13.3).
Table 13.3
Clinical features associated with functional NETS
Tumour | Clinical features |
|---|---|
Carcinoid | Flushing, diarrhoea, nausea, vomiting, abdominal pain, bronchoconstriction, fibrosis of the tricuspid and pulmonary valves |
Insulinoma | Confusion, sweatiness, dizziness, loss of conscious, relief with eating |
Gastrinoma | Zollinger-Ellison syndrome; severe peptic ulceration, diarrhoea |
Glucagonoma | Weight loss, diabetes mellitus, stomatitis, diarrhoea, necrolytic migratory erythema |
VIPoma | Verner-Morrison syndrome; profuse watery diarrhoea, hypokalaemia |
Somatostatinoma | Cholelithiasis, weight loss, diarrhoea, steatorrhoea, diabetes mellitus |
Carcinoid syndrome, arising from the release of serotonin and other vasoactive mediators, occurs in up to 35 % of patients with NETS [49]. Symptoms include flushing, diarrhoea, abdominal pain and those arising from cardiac involvement. Pancreatic NETs are functional in approximately 40 % of cases, leading to a variety of symptoms [50] (Table 13.3). When endocrinopathies do occur, a palliative liver resection can frequently treat these symptoms very effectively. In addition to liver resections providing symptomatic relief, control of associated endocrinopathies may also improve overall survival. For example, carcinoid-related valvulopathies can lead to fatal cardiac failure, insulinomas may cause life-threatening hypoglycaemia, and gastrinomas can lead to gastrointestinal perforation or massive haemorrhage. Whilst palliative liver resections may improve survival by reducing the frequency of fatal endocrinopathies, the magnitude of this effect is difficult to determine. The main factor contributing to enhanced survival is likely to be the cytoreductive effect of liver resection.
NETs possess a number of key biological features that distinguish them from most other tumour types and justify the consideration of palliative liver resections [51]. NETs characteristically possess a relatively low proliferation index, and when distant metastases do occur, these are usually limited to the liver. Even in the presence of extensive liver metastases, the primary tumour often remains resectable. In addition, tumour volume correlates well with the severity of endocrine symptoms giving predictable effects from palliative resections. The favourable biological features of NETs manifest clinically in terms of survival. Survival from metastatic NETs far exceeds that associated with an equal tumour volume arising from a metastatic gastrointestinal adenocarcinoma. These biological and clinical features result in NELM being the predominant indication for a liver resection in the palliative setting.
Where major palliative surgery is planned with the primary aim of symptomatic relief, the morbidity and mortality associated with the procedure are of paramount importance. As palliative liver resections for NELM are also associated with prolonged survival in addition to relief of associated symptoms and liver resection is associated with low rates of mortality in high volume centres, the procedure is clearly justified.
13.2.1.2 Surgical Considerations
Most authors advocate cytoreductive hepatic surgery when at least 90 % of the bulk of the tumour can be resected which is very likely to yield a successful outcome [52, 53]. In synchronous disease, concurrent resection of the primary tumour, along with hepatic metastases, can be undertaken safely in selected patients [54–56]. In the palliative setting particularly, where patients should undergo the minimum possible number of surgical procedures, adjuncts to resection can be helpful (vide infra). Successful palliative outcomes can be obtained in those with bilobar NELM [57]. Every case must be assessed on an individual basis, considering the potential benefits from surgery, along with the patients’ comorbidities and the risks involved.
Symptoms associated with functional NETs must be controlled prior to surgical intervention to reduce the risk of complications. All patients with carcinoid syndrome must receive prophylactic administration of a somatostatin analogue to prevent potential carcinoid crisis, characterised by profound flushing, bronchospasm, tachycardia and a labile blood pressure. This may be precipitated by anaesthetic induction or handling of the tumour intraoperatively. The use of short-acting octreotide is recommended, which is administered as a constant intravenous infusion. It is initiated at least 12 h prior to surgery and continued for up to 48 h postoperatively [50]. Similar prophylactic measures may be required for the problems associated with pancreatic NETs, for example, a glucose infusion for insulinomas and potassium replacement therapy for VIPomas.
13.2.1.3 Liver Resection: Results
To date, no prospective trials have been conducted to determine the results of palliative liver resections for NELM. A number of retrospective studies have attempted to assess the results of liver resections for NETs; however, frequently these trials have included both curative and palliative procedures. Primarily due to the small number of patients involved in these studies, outcome measures for the two groups are often not reported separately. Table 13.4 shows studies which detail outcome measures, in terms of either endocrine symptoms or survival, specifically for cohorts of patients who have undergone palliative liver resections.
Table 13.4
Cohorts of patients who have undergone palliative liver resections for neuroendocrine tumours
Author | Number | Primary neuroendocrine tumour | Ablative techniques in addition to liver resection | Endocrine symptoms (%) | Proportion of endocrine symptoms relieved (%) | 30-day mortality (%) | Survival, months |
|---|---|---|---|---|---|---|---|
McEntee et al. [58] | 20 | GI tract, pancreas | None | 80 | 50, complete relief | – | Mean, 11 |
Grama et al. [59] | 5 | Functional pancreatic tumours | None | 100 | NR | NR | Median, 36 |
Que et al. [53] | 46 | GI tract, pancreas | None | 91 | 83, complete relief | 4 | Median, 23.6 |
Chamberlain et al. [60] | 6 | GI tract, pancreas, lung, unknown | None | NR | 100 | NR | Median, 66 |
Nave et al. [61] | 21 | GI tract, pancreas, lung | None | NR | NR | 0 | 26 % alive at 5 years |
Chung et al. [54] | 29 | GI tract, pancreas, lung, thyroid | Cryosurgery, RFA | 100 | 90, complete relief | 3 | 31 % 5 years survival |
Sarmiento et al. [62] | 95 | GI tract, pancreas, unknown | None | NR | NR | NR | Median,16 |
Osborne et al. [55] | 23 | GI tract, pancreas, lung, unknown | RFA | 100 | NR | 4 | Mean, 32 |
Jensen et al. [63] | 13 | GI tract, pancreas, unknown | RFA | 100 | 32, complete relief; 68, partial relief | NR | NR |
Hibi et al. [64] | 7 | GI tract, pancreas, lung, unknown | RFA, ethanol injection | 86 | 83, complete relief; 17, partial relief | 0 | 0 % 5-year survival |
Mayo et al. [65] | 65 | GI tract, pancreas, lung, unknown | Yes | NR | NR | NR | Median, 77.5 |
Saxena et al. [66] | 26 | GI tract, pancreas, lung, uterus, gall bladder, unknown | Cryosurgery | NR | NR | NR | Median, 27 |
Cusati et al. [67] | 32 | Non-functional pancreatic tumours | RFA | 0 | – | 0 | 93.8, 48.1 and 32.1 % alive at 1, 5 and 10 years, respectively |
Palliative liver resections can partially or completely relieve systemic endocrine-related symptoms in more than 80 % of patients with NELM [54, 56, 60, 63, 64]. Although variability does exist in the definition of symptom relief between studies, overall the literature does suggest excellent palliation of hormone-related symptoms. Over a decade ago, Que et al. showed similar initial symptomatic response rates in patients undergoing liver resections for NELM regardless of whether these procedures were undertaken with curative or palliative intent [56]. The distinguishing feature was the earlier recurrence of symptoms in patients who had undergone palliative rather than curative resections (11.3 vs. 20.4 months). Palliative resections were undertaken if the primary tumour and 90 % or more of the hepatic disease were deemed resectable. Although few other studies specifically report the duration of symptom relief in those undergoing palliative resections, where reported the figures are similar to those obtained by Que et al. [54, 58].
The 5-year survival of patients with NELM treated with only medical therapy is 20–40 % [68, 69]. Sarmiento et al. report one of the largest single-institution studies of hepatic resections for NELM [62]. This series of 170 patients revealed 5- and 10-year survival rates of 61 and 35 %, respectively. However, curative resections were undertaken in 44 % of patients, and the survival figures provided do not distinguish between those procedures undertaken with curative and palliative intent. Considering palliative resections only, up to 48 % of patients survive for 5 years [67]. Table 13.4 highlights the inter-study variability in survival figures for palliative resections. This variability reflects differences between studies in terms of the types of tumours included and the criteria for palliative resections. For example, Hibi et al. report a 5-year survival rate of 0 % following palliative resections for NELM, but this group consists of a disproportionate number of patients with lung and pancreatic NETs, which have comparatively poor survival outcomes [64]. Overall, current evidence suggests that palliative resection for NELM does prolong survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






