Fig. 7.1
Multiple variations of common hepatic bile duct anatomy. (a) Normal anatomy of the hepatic bile duct confluence. (b) Trifurcation type anatomy of the left hepatic with the anterior and posterior sectoral ducts. (c) Right anterior duct (c1) and posterior duct (c2) with common hepatic bile duct drainage. (d) Aberrant drainage of right sectoral ducts into left hepatic duct. (e) Absence of normal hepatic confluence. (f) Absence of right hepatic duct, with right posterior draining into cystic duct
Table 7.1
Endoscopic retrograde cholangiopancreatography (ERCP) estimated frequency of complications, risks, and consequences
Complications, risks, and consequences | Estimated frequency |
|---|---|
Most significant/serious complications | |
Infectiona | |
Cholangitisa | 1–5 % |
Intra-abdominal (abscess or free perforation) | 0.1–1 % |
Systemica | 0.1–1 % |
Bleedinga | |
Sphincterotomy site | 1–5 % |
Bile duct | 0.1–1 % |
Perforationa | |
Esophagus | <0.1 % |
Stomach | <0.1 % |
Duodenum | 0.1–1 % |
Bile duct | <0.1 % |
Failure rate of ERCP (not technically possible to complete ERCP)a | 1–5 % |
Failure to visualize the ampullaa | 1–5 % |
Failure to adequately biopsy lesion(s)a | 1–5 % |
Failure to stenta | 1–5 % |
Failure to image the ducta | 1–5 % |
Failure to adequately drain the bile ducta | 1–5 % |
Pancreatitisa | 1–5 % |
Rare significant/serious problems | |
Bile leak | 0.1–1 % |
Fistula | 0.1–1 % |
Injury to mouth, teeth, pharynx, or larynx | 0.1–1 % |
Aspiration pneumonitis | 0.1–1 % |
Biliary obstructiona | 0.1–1 % |
Ampullary stenosisa | 0.1–1 % |
Stent migrationa | 0.1–1 % |
Multisystem organ failurea | 0.1–1 % |
Deatha | <0.1 % |
Less serious complications | |
Paralytic ileus | 0.1–1 % |
Blood transfusion | <0.1 % |
Perspective
See Table 7.1. Post-ERCP pancreatitis remains the major source of morbidity. The severity of this pancreatitis can be significant, requiring surgical debridement and drainage. The three most common risk factors for post-ERCP pancreatitis remain sphincter of Oddi dysfunction, difficult cannulation, and previous sphincterotomy. In most patients, post-ERCP pancreatitis is actually transient hyperamylasemia and is self-limited, requiring only IV medications and supportive care while patients are in the hospital. This complication can be devastating in the patient with a surgically resectable pancreatic cancer that can be made unresectable following ERCP, with pancreatic duct cannulation and contrast injection. Thus, the decision for pancreatic duct cannulation and contrast injection should be made judiciously. The fundamental philosophy of ERCP is that it is most dangerous for patients who need it least. MRI imaging of the cholangiopancreatic system (MRCP) offers an alternative in some cases. Bleeding is rarely severe, but this is a small but important risk of ERCP. Failure to cannulate the CBD or pancreatic duct or failure to obtain a satisfactory representative biopsy can depend on the pathology, but may significantly influence diagnosis and decision-making.
Major Complications
The most common complication remains pancreatitis, with an overall incidence (depending on the patient indication/selection) of about 6.7 %. This complication can occur after any form of ERCP, but it most commonly follows pancreatic duct cannulation and radiographic dye injection. Other causes of post-ERCP pancreatitis include distal bile duct balloon dilatation, which leads to swelling of the sphincter and transient occlusion of the distal pancreatic duct. Various methods have been utilized to prevent this complication, with the most common being the use of a transient pancreatic duct stent. This prevents distal pancreatic duct obstruction and reduces the incidence of post-ERCP pancreatitis following this form of distal bile duct dilatation. More common is transient hyperamylasemia, which occurs in 40–75 % of patients, but is self-limiting and usually asymptomatic. Duodenal perforation is another serious complication related to difficulty in passing the side-viewing endoscope through the pylorus and past the duodenal bulb into the second portion of the duodenum. This complication can lead to sepsis from intraperitoneal or retroperitoneal spillage. Treatment is defined by the extent of leakage and the patient’s clinical presentation. In a small subset of patients, a retroperitoneal duodenal perforation can be managed nonoperatively with IV antibiotics and supportive care. Otherwise, open surgical intervention to repair the duodenum with possible duodenal exclusion may be indicated. Wound drainage is usually required. Bleeding from the sphincterotomy site is another form of serious complication. This most commonly occurs when the incision is used to make a sphincterotomy at the 1–2 o’clock position of the sphincter, instead of the 11 o’clock position. This failure to properly place the incision can lead to significant hemorrhage from the gastroduodenal artery. In anticoagulated patients, risk is considerable and reversal may avoid bleeding. Cholangitis can occur with instrumentation of the bile duct; however, it is usually adequately treated with IV antibiotics provided there is adequate biliary drainage. Infection is usually secondary to one of the complications above, but may be severe and is a major cause for morbidity, multisystem organ failure, and even mortality.
Consent and Risk Reduction
Main Points to Explain
Risk of perforation/leakage/fistula
Infection
Bleeding
Risk of failed diagnosis
Risk of injury to mouth/teeth
Risk of open operation
Risk of further surgery
Open Choledochostomy and Choledochoscopy (Rigid or Flexible)
Description
General anesthesia is used. Choledochoscopy is usually performed with removal of the gallbladder. Open choledochoscopy is nowadays very rarely performed alone as a separate procedure after cholecystectomy for later diagnosis of common bile duct stones. Alternative, closed methods (usually ERCP) or minimally invasive common bile duct (CBD) exploration are usually preferable options. The aim is similar to choledocoscopy when performed with cholecystectomy, that is, to inspect the distal common bile duct (CBD), the proximal hepatic duct, and sectoral ducts. Cholangiography may be used first to fill the CBD with radiopaque contrast material to show the ductal anatomy (showing constrictions, branching, and duct size), any filling defects (usually calculi), and to indicate the drainage pattern of the duct. CBD scope use was initially defined by Bakes in 1923. Rigid choledochoscopes with varying degrees of angulation allowed visualization with limited optics and accessibility. Flexible choledocoscopy, in 1976, permitted better and easier vision. Choledochostomy is a fundamental maneuver in biliary surgery, primarily in the management of common bile duct stones and bile duct obstruction. The technique of choledochostomy usually follows an open cholecystectomy and open choledochotomy. It is preferable that the larger 5 mm flexible choledochoscope is attached to a monitor, with a 2 mm working instrument channel. The scope is connected to a pressure irrigation system for adequate visualization. The scope is introduced through an 8–10 mm longitudinal choledochotomy in the common bile duct. Slow circumferential inspection of the distal common bile duct then proceeds down to and through the sphincter into the second portion of the duodenum. The instrument is reversed and the common hepatic duct, the left hepatic duct, and the right anterior and posterior sectoral ducts should be inspected. Gallstones are usually visualized easily. Most are “free floating” in the common bile duct because of the constant irrigation. Some will easily pass into the duodenum because of the pressure of the irrigation system. Others may be found in the ampulla, embedded in the duodenal wall, or in a diverticulum of the distal duct. For the free-floating stone that does not pass into duodenum, either a basket or the balloon can be utilized for extraction of the stones through the choledochotomy. Some choledochoscopy instrument sets also include forceps that can be helpful in the removal of an impacted stone. Other techniques that can be used are the administration of IV glucagon, as well as a gentle dilatation of the ampulla with the choledochoscope. In the presence of multiple small calculi, either in the distal common bile duct or, more commonly, within the hepatic ductal system, biliary-enteric bypass should be considered.
Anatomical Points
The anatomical pattern is partly dependent on the natural anatomy and also on any previous surgery. The variations in natural anatomy are described below for other procedures. Previous cholecystectomy may lead to distortion of the duct due principally to scarring and adhesions. Primary dissection to identify the anterior surface of the common bile duct can be difficult in a small subset of patients who have variant biliary anatomy in the form of an early right posterior sectoral duct takeoff, a long tortuous cystic duct, or an early bifurcation of the common bile duct. This variance in biliary anatomy can lead to significant biliary damage due to inadvertent ligation or improper identification, which can lead to a choledochotomy, performed in a variant bile duct that is too small for cannulation. Surrounding organs (e.g., colon, small bowel, liver, duodenum, and stomach) may be adherent to the hilar structures including the CBD, increasing risk of injury. An enlarged liver or narrow costal angle may impede access.
Table 7.2
Open choledochostomy and choledochoscopy (rigid or flexible) estimated frequency of complications, risks, and consequences
Complications, risks and consequences | Estimated frequency |
|---|---|
Most significant/serious complications | |
Infectiona | 1–5 % |
Subcutaneous | 1–5 % |
Intra-abdominal/pelvic | 0.1–1 % |
Cholangitisa | 1–5 % |
Abscessa | 0.1–1 % |
Systemica | 0.1–1 % |
Bleeding/hematoma formationa | 1–5 % |
Failure rate (not technically possible to complete choledochoscopy)a | 1–5 % |
Failure to reach the ampullaa | 1–5 % |
Failure to visualize duct/remove calculi from the ducta | 1–5 % |
Rare significant/serious problems | |
Perforation or injury (laceration/dissection)a | |
Bile duct, ampulla, duodenum, small bowel, colon | 0.1–1 % |
Small bowel obstruction (early or late)a [Adhesion formation] | 0.1–1 % |
Aspiration pneumonitis | 0.1–1 % |
Biliary/pancreatic leaka | 0.1–1 % |
Pancreatitisa | 0.1–1 % |
Biliary obstructiona | 0.1–1 % |
Late bile duct/ampullary stenosisa | 0.1–1 % |
Fistula (duodenal/biliary/pancreatic)a | <0.1 % |
Multisystem organ failurea | 0.1–1 % |
Deatha | <0.1 % |
Less serious complications | |
Pain/tenderness | |
Acute (<4 weeks) | >80 % |
Chronic (>12 weeks) | 1–5 % |
Paralytic ileus | 5–20 % |
Incisional hernia formation (delayed heavy lifting/straining) | 0.1–1 % |
Nasogastric tubea | 1–5 % |
T-tube biliary drainagea | >80 % |
Wound scarring (poor cosmesis) | 1–5 % |
Wound drain tube(s)a | 1–5 % |
Blood transfusion | <0.1 % |
Perspective
See Table 7.2. The importance of endoscopic intraluminal inspection of the extrahepatic biliary system cannot be overemphasized. Choledochoscopy remains the most accurate method to detect and remove bile duct stones. Various debates in regard to the percentage of remaining stones following either open cholecystectomy or laparoscopic cholecystectomy further emphasize the need for choledochoscopy. All agree that choledochoscopy can result in the recovery of additional stones in upwards of 10–15 % of patients following standard attempts of stone extraction. Because of the significant rise in the incidence of laparoscopic cholecystectomy, the performance of open cholecystectomy, with open choledochostomy and operative removal of common bile ducts, has decreased significantly in the last 5–10 years. This has placed greater emphasis on surgical programs creating structured training courses on animal models so that the technique of open cholecystectomy, open choledochotomy, and choledochoscopy can be performed safely and proficiently. The ability to perform these procedures at the initial operation can prevent significant postoperative morbidity and chronic biliary instrumentation in these patients. The major chronic disability that can occur after a common bile duct exploration is damage and stricture to the extrahepatic biliary system. This primarily occurs with inadequate exposure of the common bile duct, incomplete identification of the common bile duct, or failure to recognize a small diameter common bile duct. Further surgery for drainage may be required. Persistent bile leakage, bile peritonitis, and/or fistula formation are rare, but potentially serious complications that may require further surgery. Inadvertent T-tube dislodgement or removal may delay recovery. Arterial injury and bleeding is also another significant risk factor related to anatomical point, primarily in jaundiced patients who undergo this procedure. Careful dissection, as well as visualization of either an accessory or replaced right hepatic artery, cannot be overemphasized. Bile leak is very common in the immediate postoperative period from closure of a choledochotomy or from the raw liver. This type of bile leak is often inconsequential and almost always resolves via the drain tube or spontaneously and seldom requires reoperation. The need for radiological cannulation of a bile collection is rare. Infection is usually transient and limited or treated with antibiotics. Severe systemic sepsis is life threatening, but rare, and usually associated with established preoperative sepsis. Failure to visualize the bile duct adequately occurs infrequently, but may dictate an alternative approach.
Major Complications
Bile duct injury is a major complication related to a CBD exploration. Primary dissection to identify the anterior surface of the CBD can be difficult in a small subset of patients who have variant biliary anatomy in the form of an early right posterior sectoral duct takeoff, a long tortuous cystic duct, or an early bifurcation of the CBD. These variations in biliary anatomy can lead to significant biliary damage because of inadvertent ligation or transection or choledochotomy performed in portion of the bile duct too small for primary closure or even exploration. Bile duct perforation or mucosal tears may result from placement of the choledochoscope into a bile duct that is too small or into a variant biliary duct. If perforation is appreciated at the time of choledochotomy, primary repair may not provide a long-term effective solution and hepaticojejunostomy should be considered. The incidence of biliary stricture after undergoing a biliary exploration is usually small, but when this complication develops it is significant, often requiring further surgery, sometimes including biliary bypass procedures. Stricture results from bile duct trauma, either from chronic choledocholithiasis or iatrogenic from choledochotomy, choledochoscopy, instrumentation or inadvertent laceration, or ligation. As described above this can occur at any level in the biliary tree and may only become evident years after biliary surgery. Damage of the ampulla can result from chronically impacted CBD stones at the ampulla, causing edema and mucosal irritation. After the removal of an impacted CBD stone, further instrumentation of the ampulla with the choledochoscope can lead to mucosal tears, which can lead to long-term stricture formation and require further biliary instrumentation for drainage. Bleeding during dissection of the CBD can occur. A right hepatic artery can transverse anterior to the bile duct, which can be injured or ligated during this dissection. The portal vein should also be properly identified on the medial-posterior surface of the CBD to ensure that only anterior dissection of the common bile duct is performed prior to choledochotomy. Missing a retained stone due to patient anatomy, inflammation, or ductal diverticula can occur causing poor visualization of the distal CBD, CHD, and sectoral hepatic ducts. Improper use of the choledochoscope or inadequate flow of irrigation is possible technical contributors to this. This can lead to the retention of CBD stones, which can lead to later cholangitis. Further biliary instrumentation by ERCP, percutaneous transhepatic cholangiography, or repeat surgical exploration may be required. Cholangitis prior to or at surgery or postoperatively can result in severe sepsis and multisystem organ failure and is the major cause of mortality when it occurs.
Consent and Risk Reduction
Main Points to Explain
Risk of leakage/fistula
Pancreatitis
Infection
Bleeding
Risk of organ injury
Risk of further surgery
Open Cholecystectomy (Without Common Bile Duct Exploration)
Description
General anesthesia is used. The aim of the procedure is to remove the gallbladder (GB) and its contents. The “retrograde” technique is defined by the initial dissection of the hilar structures of the GB within Calot’s triangle, then dissecting toward the fundus. The “anterograde or antegrade” (fundus down) technique is defined by removal of the GB from the liver bed first, before transection of the cystic duct and artery. The operation is usually performed through either a right subcostal, right upper transverse, or an upper midline incision. After adequate exposure is obtained, the use of the retrograde or anterograde technique depends on surgeon preference, as well as the degree of inflammation present. If significant GB inflammation is present, the anterograde technique can avoid inadvertent biliary or arterial injury and is also usually easier, along an “inflammatory plane” around the GB. The three integral steps in performing an open cholecystectomy are (1) identification/ligation/division of the cystic duct near the GB infundibulum, (2) identification/ligation/division of the cystic artery distal to the right hepatic artery, and (3) removal of the GB from the liver bed with meticulous hemostasis. An inflamed GB can often be “pinched” from the liver bed. A drain can be left in patients in whom significant inflammation/ooze is encountered.
Anatomical Points
Variations in biliary ductal anatomy are numerous (Fig. 7.1), and a surgeon should be aware of these to prevent biliary injury and subsequent stricture during an open cholecystectomy. A majority have the standard bifurcation of the common hepatic duct, with a trifurcation of the common hepatic duct being the second most common. In almost all cases, the posterior biliary segments lie more laterally than the anterior segments, such that in the evaluation of cholangiograms, the segment 6 and 7 ducts are seen inferio-laterally and superio-laterally, respectively. Other common radiographic presentations show the posterior sectoral ducts arching close to the confluence of the common hepatic duct. In addition, a right sectoral duct can cross to the left and join the left hepatic duct in 28 % of patients; in 22 %, this is the posterior sectoral duct, and in 6 %, it is the anterior sectoral duct. Hepatic arterial anatomic point should also be considered when performing any type of biliary surgical exploration and/or instrumentation (Fig. 7.2). In 25 % of patients, the right hepatic artery will either be completely replaced or have a large accessory branch from the superior mesenteric artery. In addition, the left hepatic artery can be completely replaced or have a large accessory branch from the left gastric artery through the lesser omentum. In other less common variances, the left and right can originate from the celiac trunk or branch from a very short common hepatic artery. The least common variance is origination of the gastroduodenal artery from the right hepatic artery. Hepatic portal anatomic point should also be considered when performing any type of biliary exploration and/or instrumentation (Fig. 7.3). Knowledge and proper identification of the coronary vein cannot be overemphasized since this can lead to substantial blood loss while attempting to gain exposure of the medial aspect of the common bile duct. The course of the portal vein (PV) is behind the common bile duct and the common hepatic duct as it approaches the liver. It then bifurcates into right PV and smaller left PV. The LPV will remain extrahepatic for a longer length behind the left hepatic artery until it enters the umbilical fissure. The RPV is a very short structure that will branch quickly into the right anterior and right posterior sectoral branches. Another main variant is division of the portal vein more proximally with the right anterior and the right posterior branching independently (Fig. 7.3).
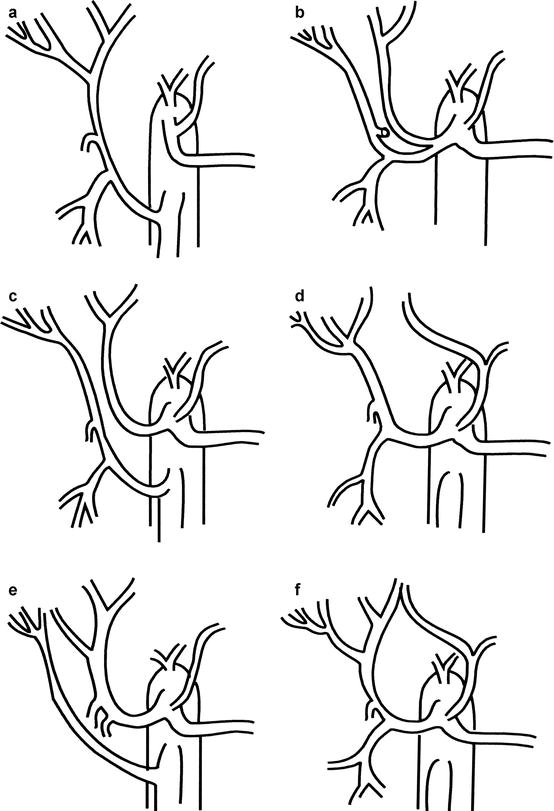
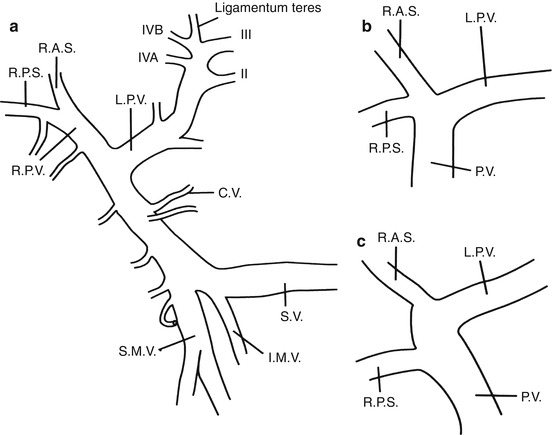

Fig. 7.2
Hepatic arterial anatomic point is common, with over 25 % of patients having either a partial or complete replacement of the right hepatic artery from the superior mesenteric artery (a, c, e). In addition, the left hepatic artery may either be partially or completely replaced from the left gastric artery (d, f). In a small subset of patients, the left or right hepatic artery may arise from the celiac axis (b, c). Lastly, the gastroduodenal artery may arise from the right hepatic artery (b, c)

Fig. 7.3
Portal venous anatomic point is less common, with the most common venous anatomy having (a) the portal vein (PV) arise from the confluence of the superior mesenteric vein (SMV) and the splenic vein (SV), with the inferior mesenteric vein (IMV) draining into the SV. (b) Another anatomic point may arise from the lack of a true right portal vein with the right posterior sectoral (RPS) and the right anterior sectoral (RAS) arising from a common trunk with the left portal vein (LPV). (c) Lastly, the division of the right portal vein may occur more proximally with the RPS arising independently from the portal venous trunk
Table 7.3
Open cholecystectomy (without common bile duct exploration) estimated frequency of complications, risks, and consequences
Complications, risks and consequences | Estimated frequency |
|---|---|
Most significant/serious complications | |
Infectiona overall | 1–5 % |
Subcutaneous | 1–5 % |
Intra-abdominal/pelvic | 0.1–1 % |
Systemic | 0.1–1 % |
Bleeding/hematoma formationa | 1–5 % |
Wound/intra-abdominal | |
Rare significant/serious problems | |
Failure to detect/remove calculia | 0.1–1 % |
Pancreatitis/pancreatic injury/pancreatic cyst/pancreatic fistulaa | 0.1–1 % |
Seroma formation | 0.1–1 % |
Injury to the bowel or blood vesselsa | 0.1–1 % |
Duodenal/gastric/small bowel/colonic | |
Vascular injurya | 0.1–1 % |
Liver injury | 0.1–1 % |
Bile duct injury/bile leak/collection/fistulaa | 0.1–1 % |
Jaundice (dislodgement of gallstones into common bile duct)a | 0.1–1 % |
Small bowel obstruction (early or late)a | 0.1–1 % |
Operative cholangiogram complicationsa | |
Dye reaction/cholangitis/pancreatitis/radiation exposure | <0.1 % |
Possibility of colostomy/ileostomy (very rare)a | <0.1 % |
Multisystem organ failure (renal, pulmonary, cardiac failure)a | 0.1–1 % |
Deatha | <0.1 % |
Less serious complications | |
Pain/tenderness | |
Acute (<4 weeks) | >80 % |
Chronic (>12 weeks) | 1–5 % |
Paralytic ileusa | 50–80 % |
Wound dehiscence | 0.1–1 % |
Muscle weakness (atrophy due to denervation esp. Kocher’ incision) | 1–5 % |
Wound scarring (poor cosmesis/wound deformity) | 1–5 % |
Incisional hernia formation (delayed heavy lifting/straining) | 0.1–1 % |
Nasogastric tubea | 1–5 % |
Wound drain tube(s)a | 1–5 % |
Perspective
See Table 7.3. Laparoscopic cholecystectomy has made open cholecystectomy rare. Surgeons nowadays often receive little exposure to open cholecystectomy. Knowledge and the technical expertise in performing the occasional open cholecystectomy cannot be overemphasized. However, there is often little variation on the indications for initial cholangiography during cholecystectomy by either the open and laparoscopic approaches. When poor access or GB inflammation may not allow laparoscopic exposure, open cholecystectomy should be regarded as a safe alternative, not a failed laparoscopic cholecystectomy. Open cholecystectomy still remains an important, essential technique for patients in whom a laparoscopic cholecystectomy cannot be safely performed. Whether complications of open cholecystectomy are higher through relative surgeon, inexperience is controversial. The main risks are bile duct injury, including transection, and ligation and bleeding from the liver or hepatic vascular system or from other organs, omentum, or abdominal wall. Retained calculi are another complication.
Major Complications
A rare, but serious complication that can occur during an open cholecystectomy is common bile duct ligation/injury. If a common bile duct injury is identified intraoperatively, primary repair is almost always associated with chronic biliary stricture and inadequate biliary drainage. The universally accepted technique for managing common bile duct injury is either a choledochoduodenostomy (for distal common bile duct injury) or a hepaticojejunostomy (for common hepatic duct injury). Injury of a small diameter (less than 5 mm) low posterior sectoral bile duct takeoff (anatomic point) can be managed with proximal ligation alone, but if the duct diameter is larger, then biliary reconstruction with either the duodenum or with a Roux-en-Y of jejunum is indicated. Recent reports have demonstrated that immediate intraoperative reconstruction for major bile duct injury will lead to an equivalent quality of life when compared to patients who underwent an uncomplicated cholecystectomy. Arterial injury inadvertently of the right hepatic artery or the right posterior sectoral artery can occur causing hemorrhage, and ligation of the hepatic artery reduces local hepatic blood flow. These complications may be avoided by careful dissection close to the infundibulum of the GB. In a normal patient without hyperbilirubinemia, attempts at reconstruction should not be made and the surgeon should ensure that there is adequate ligation. If, however, this complication occurs in a patient with underlying liver failure, or hyperbilirubinemia, then there should be precise evaluation to determine whether arterial reconstitution can be achieved to avoid postoperative liver ischemia. This complication can be devastating to these types of patients because of their inability to tolerate an additional insult of arterial ischemia in the face of hyperbilirubinemia. Postoperative bleeding can result from a posterior cystic artery or bleeding from the liver bed. Precise dissection during removal of the GB and identification and ligation of a possible posterior cystic artery should always be performed during open cholecystectomy. Hemostasis of the liver bed is best achieved using initial pressure on a dry pack, but also by either staying in the subserosal plane during removal of the GB or with the use of diathermy or argon beam coagulation after removal. Injury to the portal vein, vena cava, or liver parenchyma is very uncommon, but is a serious complication when it occurs. Injury to the colon, antrum of the stomach, small bowel, or more commonly the second portion of the duodenum can occur especially with dissection of dense adhesions from GB wall inflammation. Immediate primary repair, depending on the size and extent of injury, is indicated if this occurs. A biliary leak (BL)/collection and/or biliary fistula can occur because of either dislodgement of the ligature on the cystic duct, failure to identify an accessory cystic duct at operation, or superficial liver injury causing peripheral bile duct leakage. The management of these types of injuries is primarily dictated by symptoms and/or the volume of BL via an intraoperative drain or on CT/US imaging postoperatively. Asymptomatic BL < 200 ml/day needs observation alone, since most of these resolve. When BL > 200 ml/day and persistent, US-guided percutaneous drainage, percutaneous transhepatic cholangiography, or ERCP, and drainage are often required.
Consent and Risk Reduction
Main Points to Explain
Infection
Bleeding
Risk of organ injury
Risk of leakage/fistula
Pancreatitis
Risk of further surgery
Laparoscopic Cholecystectomy (Without Common Bile Duct Exploration)
Description
General anesthesia is used for this procedure with the aim of removing the gallbladder (GB) with a minimally invasive technique. The variations in this technique primarily relate to the number and size of abdominal ports used and patient positioning. Typically four ports are placed: an umbilical 10 or 12 mm port for the camera; a 10 mm epigastric port 4 cm below the xiphoid process, entering to the right side of the falciform ligament; and two 5 mm trocars placed at the midclavicular line just above the umbilicus and the anterior axillary line 4–5 cm below the costal margin, respectively. These enable manipulation of the GB. In this way, the fundus (tip) of the gallbladder can be retracted up and over the liver and the infundibulum can be extracted at a 90° angle to the common bile duct. A CO2 gas pneumoperitoneum is maintained at approximately 12–15 mmHg intra-abdominal pressure, and dissection of Calot’s triangle close to the GB is commenced. Both the cystic artery and the cystic duct are identified close to the GB and then ligated using clips. The common bile duct and the common hepatic duct may be identified (not always feasible) prior to ligation and transection of the cystic duct. The gallbladder is dissected, usually retrogradely, in a subserosal plane using diathermy cautery. Image intensification, and intraoperative cholangiography via the cystic duct or GB, may be useful to define both anatomy and detect calculi, prior to division of the cystic duct. In the presence of inflammation or oozing, a temporary drain may be used via one of the 5 mm trocar port sites.
Anatomical Points
Variations in biliary ductal system are numerous (Fig. 7.1) and require explanation to reduce biliary injury and subsequent stricture during an open cholecystectomy. The majority of anatomic points have a standard bifurcation of the common hepatic duct, with a trifurcation of the common hepatic duct being the second most common. In almost all cases, the posterior biliary segments lie more laterally than the anterior segments, such that in the evaluation of cholangiograms the segment 6 and 7 ducts are seen inferio-laterally and superio-laterally, respectively. Other common radiographic presentations are for the posterior sectoral ducts to be seen arching close to the confluence of the common hepatic duct. In addition, a right sectoral duct can cross to the left and join the left hepatic duct in 28 % of patients; in 22 %, this is the posterior sectoral duct, and in 6 % the anterior sectoral duct. Hepatic arterial anatomic point should also be considered when performing any type of biliary exploration and/or instrumentation (Fig. 7.2). In 25 % of patients the right hepatic artery will either be completely replaced or have a large accessory branch from the superior mesenteric artery. In addition the left hepatic artery can be completely replaced or have a large accessory branch from the left gastric artery through the lesser omentum. In other less common variants, the left and right can originate from the celiac trunk or branch from a very short common hepatic artery. The least common variant is origination of the gastroduodenal artery from the right hepatic artery. Hepatic portal anatomic point should also be considered when performing any type of biliary exploration and/or instrumentation (Fig. 7.3). Knowledge and proper identification of the coronary vein (CV) cannot be overemphasized, since this can lead to substantial blood loss while attempting to gain exposure of the medial aspect of the common bile duct. The course of the portal vein is behind the common bile duct and the common hepatic duct as it approaches the liver. There it usually bifurcates into a larger right (RPV) and smaller left portal vein (LPV). The LPV remains extrahepatic for a longer period behind the left hepatic artery, until it enters the umbilical fissure. The RPV is very short and branches quickly into the right anterior and right posterior sectoral branches. The one main variance is division of the portal vein more proximally with the right anterior and the right posterior branching independently (Fig. 7.3). Occasionally, hepatic vessels may lie in the gallbladder bed, where they are in danger during dissection of the GB from the liver.
Table 7.4
Laparoscopic cholecystectomy (without common bile duct exploration) estimated frequency of complications, risks, and consequences
Complications, risks, and consequences | Estimated frequency |
|---|---|
Most significant/serious complications | |
Infectiona overall | 1–5 % |
Subcutaneous | 1–5 % |
Intra-abdominal/pelvic | 0.1–1 % |
Systemic | 0.1–1 % |
Port site | 0.1–1 % |
Bleeding/hematoma formation | 1–5 % |
Wound/intra-abdominal | |
Conversion to open operation | 1–5 % |
Rare significant/serious problems | |
Failure to detect/remove calculia | 0.1–1 % |
Pancreatitis/pancreatic injury/pancreatic cyst/pancreatic fistulaa | 0.1–1 % |
Seroma formation | 0.1–1 % |
Injury to the bowel or blood vessels (trocar or diathermy)a | 0.1–1 % |
Duodenal/gastric/small bowel/colonic | |
Vascular injury | 0.1–1 % |
Liver injury | 0.1–1 % |
Bile duct injury/bile leak/collection/fistula | 0.1–1 % |
Necessity for open biliary drainage procedure(s) after duct injurya | 0.1–1 % |
Jaundice (dislodgement of gallstones into common bile duct)a | 0.1–1 % |
Small bowel obstruction (early or late)a | 0.1–1 % |
Pneumothorax | 0.1–1 % |
Deep venous thrombosis | 0.1–1 % |
Gas embolusa | 0.1–1 % |
Operative cholangiogram complications | |
Dye reaction/cholangitis/pancreatitis/radiation exposure | < 0.1 % |
Possibility of colostomy/ileostomy (very rare)a | <0.1 % |
Multisystem organ failure (renal, pulmonary, cardiac failure)a | 0.1–1 % |
Deatha | <0.1 % |
Less serious complications | |
Pain/tenderness | |
Acute (<4 weeks) | >80 % |
Chronic (>12 weeks) | 1–5 % |
Paralytic ileusa | 50–80 % |
Wound dehiscence | 0.1–1 % |
Wound scarring (poor cosmesis/wound deformity) | 0.1–1 % |
Port-site hernia formationa | 0.1–1 % |
Nasogastric tubea | 1–5 % |
Wound drain tube(s) | 1–5 % |
Perspective
See Table 7.4. The rapid acceptance of laparoscopic cholecystectomy has placed it among the most common procedures performed by the general surgeon in most medical centers. Laparoscopic cholecystectomy indications are the same as for open cholecystectomy including symptomatic cholelithiasis, acute cholecystitis, chronic cholecystitis, acalculous cholecystitis, and asymptomatic cholelithiasis in patients with sickle cell disease, chronic immunosuppression, or renal transplant and occasionally prophylactically in people travelling to isolated areas (e.g., Antarctic missions or space). Contraindications to laparoscopic cholecystectomy include inability to tolerate general anesthesia or pneumoperitoneum, refractory coagulopathy, and advanced gallbladder (GB) carcinoma. Relative contraindications have continued to change, depending upon the surgeon’s preference, but include previous upper abdominal surgery, cholangitis, early GB carcinoma, pregnancy, diffuse peritonitis, cirrhosis, portal hypertension, and severe chronic obstructive pulmonary disease (COPD). These indications, as well as contraindications, should always be considered carefully so that an adequate complication risk-versus-benefit discussion can take place with the patient. Most procedures are not associated with complications. However, the complications that can occur during a laparoscopic cholecystectomy can be severe and chronically debilitating. Complications related to trocar injury of bowel or major blood vessels (aorta, inferior vena cava, iliac vessels) or gas embolism should always be considered. Bile duct injury related to inadvertent common bile duct laceration or ligation remains a low, but severe, risk in all patients undergoing this procedure. Most injuries related to laparoscopic cholecystectomy should be repaired through open exploratory operation(s). This allows full inspection of the abdomen, especially at the trocar insertion sites, and adequate access to evaluate and deal with the problem.
Major Complications
Bile duct injuries (BDI), usually to the common bile duct, are among the most serious complications during laparoscopic cholecystectomy. The incidence of BDI (0.2–0.6 %) has been reported to be three times higher than for open cholecystectomy (0.1–0.25 %). Recent data from centers with expertise in laparoscopic cholecystectomy show low rates of BDI similar to open cholecystectomy. More importantly, the number of cases of common bile duct injury in which there is a delay in diagnosis is also increasing, which has an adverse outcome for patients (Schol et al. 1995). It is paramount to avoid bile duct injuries by risk reduction at the initial operation. Many reports have demonstrated the important techniques in prevention of this injury including dissection close to the infundibulum, extraction of the infundibulum to create a right angle to the common bile duct, and, most importantly and ideally, identification of the common bile duct prior to transection of the cystic duct. Working close to the GB, defining the GB edge, and clip ligating only definite cystic arterial and duct structures close to the GB are useful methods. Another technique, which has recently gained acceptance, is performing the procedure in a “dome-down technique” (similar to open cholecystectomy) so that proper identification of the common bile duct can be achieved once the entire GB has been removed from the liver bed. In the case of a bile duct injury, attempted primary repair of the duct is almost universally associated with chronic biliary stricture and inadequate biliary drainage. The universally accepted technique in managing an inadvertent common bile duct ligation is a choledochoduodenostomy if the injury is within the distal common bile duct, a choledochojejunostomy if the injury is higher on the common bile duct, or a hepaticojejunostomy if the injury is within the common hepatic duct. If the bile duct injury occurs because of the anatomic point of a low posterior sectoral bile duct takeoff, it can be managed by one of two techniques. If this low takeoff is small in diameter (less than 5 mm), then adequate proximal ligation should be performed and no further reconstruction is indicated. However, if the diameter of this duct is larger (>1 cm), then some form of biliary reconstruction, with either the duodenum or a Roux-en-Y of jejunum, is indicated. Immediate intraoperative repair of any major bile duct injury is usually indicated and should not normally be left to a second operation. Currently, an immediate open repair of bile duct injuries is often far easier than failed attempts at laparoscopic repairs and subsequent reoperation, but this is dictated by surgeon preference. Trocar injury is another serious complication that usually occurs primarily through the use of a Verres needle (with the closed puncture technique) or a trocar introduced without direct intra-abdominal vision. Injury to the bowel or vascular injury may result. Risk may be reduced almost completely using the “open cutdown technique” for entry into the peritoneal cavity under direct vision (Hassan technique), placement of the camera via this port, and then laparoscopic vision of puncture of the abdominal wall for the other trocars. Retractable trocars, introducing rods and sharp stylettes, can also reduce risks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




