Fig. 3.1
Solitary fibrous tumor. A primary hepatic solitary fibrous tumor on cut section is well-demarcated with a tan-white whorled cut surface
3.1.4 Microscopic Findings
Solitary fibrous tumors are composed of bland, uniform, fibroblast-like spindle cells embedded in a dense keloid-like hyalinized stroma. There can be accompanying, branching hemangiopericytoma-like (staghorn) gaping vessels (Fig. 3.2). Some lesions show alternating hypocellular and hypercellular areas without a distinct organization of the spindle cells (Figs. 3.3, 3.4, 3.5, 3.6, and 3.7). This so-called “patternless pattern” can be likened to shoaling fish, whereby fish are clustered in a homogeneous group, but swimming independently of one another in a uniformly uncoordinated way (Figs. 3.8, 3.9, 3.10, 3.11, 3.12, and 3.13). In rare cases, solitary fibrous tumors can surround a dilated bile duct (Fig. 3.14). The edges can be focally infiltrative in some cases, with entrapped islands of hepatocytes and bile ducts (Fig. 3.15).
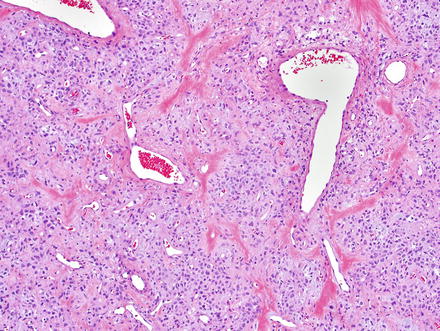
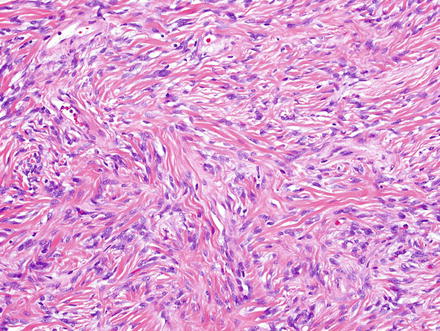
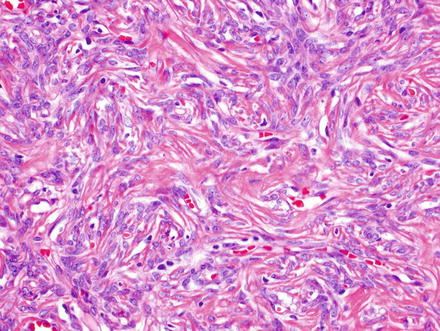
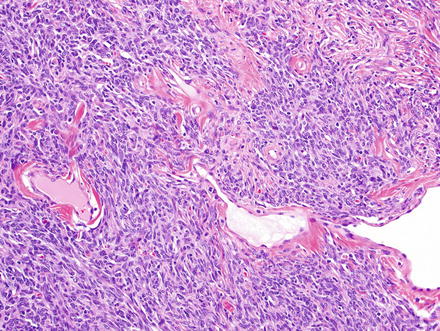
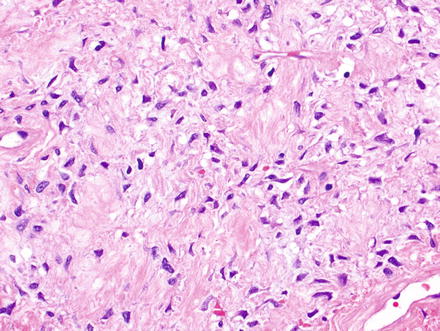
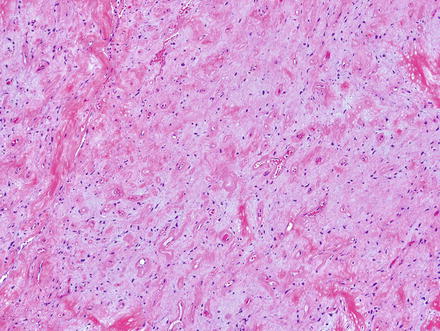
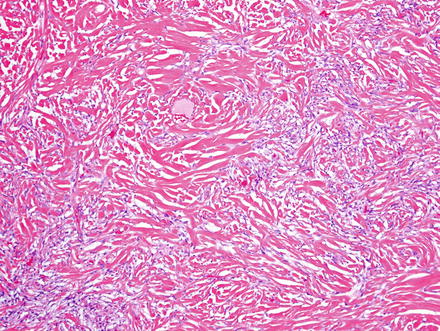
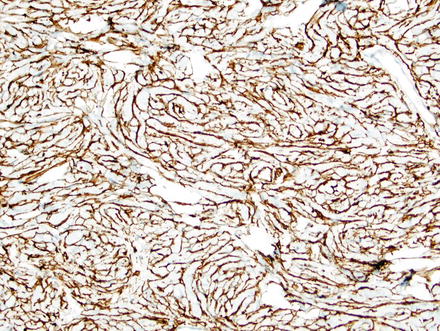
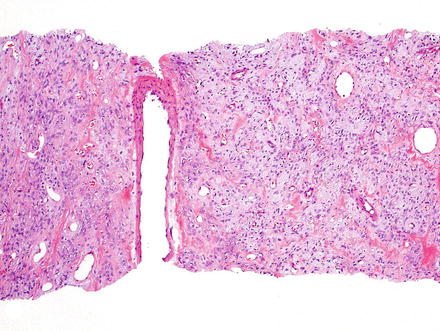
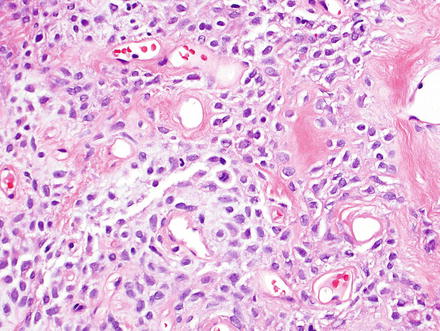
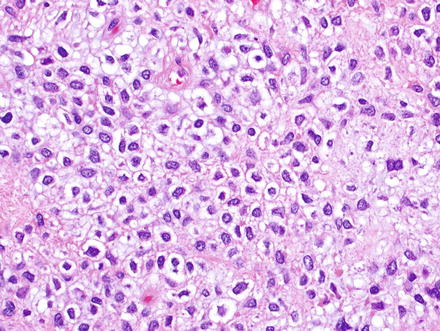
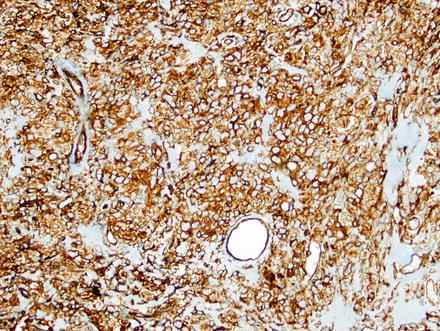
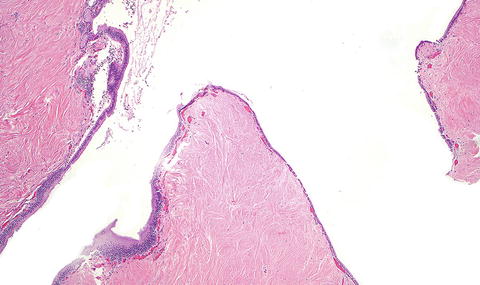
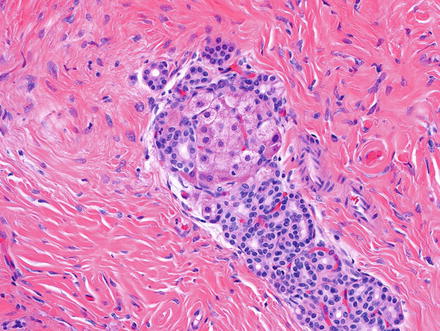

Fig. 3.2
Solitary fibrous tumor. The characteristic gaping “staghorn” vessels are surrounded by haphazardly arranged cells embedded in a densely collagenized stroma

Fig. 3.3
Solitary fibrous tumor. Fascicles of spindle cells swirl without a distinct pattern

Fig. 3.4
Solitary fibrous tumor. Some areas show a storiform arrangement of short fascicles

Fig. 3.5
Solitary fibrous tumor. Densely cellular areas can raise concern for a highly cellular sarcoma, such as synovial sarcoma

Fig. 3.6
Solitary fibrous tumor. Areas with less cellularity maintain a patternless pattern of cellular growth

Fig. 3.7
Solitary fibrous tumor. The variability in cellularity can make the diagnosis challenging, as in this area, which shows low cellularity, collagen bands, and a slightly myxoid backdrop

Fig. 3.8
Solitary fibrous tumor. This example shows low cellularity and a prominent densely collagenized backdrop

Fig. 3.9
Solitary fibrous tumor. A CD34 immunostain of the previous case highlights the spindle cells

Fig. 3.10
Solitary fibrous tumor. Needle core biopsies can be challenging, as in this case which shows a slightly myxoid backdrop

Fig. 3.11
Solitary fibrous tumor. Higher magnification of previous case shows gaping vessels, a collagen backdrop, and a fairly uniform population of cells without a specific growth pattern. A single mitotic figure (center) is seen in this field and warrants further examination for features of malignancy (necrosis, infiltrative margins, high cellularity, nuclear atypia, and >4 mitoses in 10 high-power fields)

Fig. 3.12
Solitary fibrous tumor. Epithelioid morphology of the cells can be misleading

Fig. 3.13
Solitary fibrous tumor. Application of CD34 immunostain on the previous case highlights the tumor cells

Fig. 3.14
Solitary fibrous tumor. A cystically dilated bile duct was present at the center of this solitary fibrous tumor

Fig. 3.15
Solitary fibrous tumor. Entrapped islands of hepatocytes and bile ducts are seen at the edge
Nuclei of the spindle cells are uniform and lack pleomorphism, but 10 % of these tumors may undergo malignant transformation, characterized by infiltrative margins, high cellularity, prominent cellular atypia, tumor necrosis, and increased mitotic activity (>4 mitoses per 10 high power fields) [2, 5]. Rare cases may show abrupt transition to a high-grade sarcoma.
3.1.5 Immunohistochemical Features
The tumor cells characteristically express cytoplasmic CD34 (see Figs. 3.9 and 3.13) and membranous CD99, both diffusely. Bcl-2 shows variable nuclear and cytoplasmic reactivity. Tumors should also be positive for vimentin, but negative for S100, desmin, CKIT (CD117), and cytokeratins. Smooth muscle actin (SMA) is characteristically negative, but may be weakly positive in some cases.
3.1.6 Differential Diagnosis
When found in the viscera, a significant number of other spindle cell tumors are excluded from the differential diagnosis, such as: benign fibrous histiocytoma, spindle cell lipoma, myopericytoma, and dermatofibrosarcoma protuberans, all of which have a dermal or subcutaneous location.
The remaining differential diagnosis includes gastrointestinal stromal tumor (GIST), synovial sarcoma, and Ewing sarcoma. Gastrointestinal stromal tumors have a fascicular or organoid growth pattern and are positive for CD34, CD117 and/or DOG1 in most cases. Most show KIT or PDGFRA oncogenic mutations. Synovial sarcoma has a denser population of spindle cells and a biphasic pattern and shows focal keratin positivity. Although a hemangiopericytoma-like pattern can be seen, synovial sarcoma is less vascular than solitary fibrous tumors. Additionally, synovial sarcomas show characteristic (X;18) translocations. Similarly, Ewing sarcoma contains specific translocations (most commonly 11;22) and has a much denser cellularity than solitary fibrous tumors. Ewing sarcoma is also positive for FLI-1, which is available as an immunostain at some institutions.
3.2 Lipoma
3.2.1 Definition
A lipoma is a benign tumor consisting of mature white adipocytes.
3.2.2 Clinical Features
Hepatic lipomas are uncommon tumors and are usually symptomless, regardless of their size. These lesions occur most commonly between the ages of 40 to 60 years. They are usually encountered as incidental findings on ultrasound, computed tomography, or magnetic resonance imaging. Similar to soft tissue lipomas, hepatic lipomas are found more frequently among the obese and have a significant association with hepatic steatosis [6].
3.2.3 Gross Findings
Hepatic lipomas range from a few millimeters to several centimeters in diameter and are well-circumscribed lesions with a delicate capsule. The cut surface is homogeneously yellow and has a soft greasy texture.
3.2.4 Microscopic Findings
Lipomas are composed of lobules of mature adipocytes with minimal variation in adipocyte size (Fig. 3.16). Foci of fat necrosis or hemorrhage may be present. The adipocytes show minimal size variation and have peripheral flattened nuclei without atypia (Fig. 3.17). Additional mesenchymal components may be present in some variants of the lipoma of soft tissues, including abundant fibrous tissue in fibrolipomas, cartilage in chondrolipomas, bone in the rare osteolipoma, smooth muscle in a myolipoma, and myxoid stromal change in a myxolipoma.
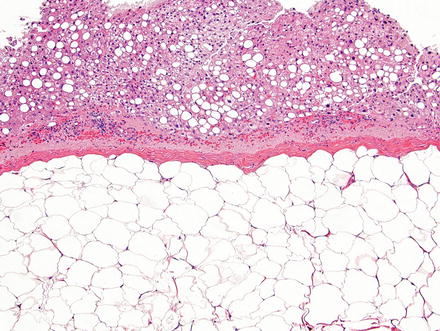
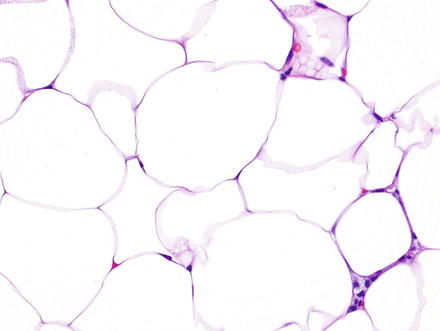

Fig. 3.16
Hepatic lipoma. The lesion is composed of a well-demarcated solid sheet of adipocytes with a delicate capsule. Note the steatosis in the background liver parenchyma, a finding commonly associated with these lesions

Fig. 3.17
Hepatic lipoma. Higher magnification of the previous case shows mature adipocytes with clear cytoplasm and flattened nuclei at the periphery lacking atypia
3.2.5 Immunohistochemical Features
Lipomas, like mature adipocytes, stain with S100 and are cytogenetically heterogeneous. Immunostaining or FISH for MDM2 can be helpful to distinguish lipoma from well-differentiated liposarcoma; MDM2 is usually amplified in liposarcomas but not in lipoma.
3.2.6 Differential Diagnosis
In the liver, the differential diagnosis includes angiomyolipoma, myelolipoma, and focal fatty change of the liver. In contrast to lipomas, which contain a homogeneous population of very uniform adipocytes, the other entities will show other elements in varying quantity. For example, the angiomyolipoma contains vessels and myoid cells in addition to fat, while myelolipomas contain hematopoietic elements. Focal fatty change of the liver is not encapsulated and is not a mesenchymal lesion of the liver. It is characterized by multiple contiguous acini showing macrovesicular steatosis of hepatocytes, and while it can appear quite fatty, the lesion is hepatocellular in origin and the presence of structures such as portal tracts, sinusoids, Ito cells, and Kupffer cells should provide clues to the diagnosis. These lesions are associated with diabetes mellitus in 45 % of cases [7]. Liposarcomas of the liver are very rare. They can be well-differentiated, but have infiltrative borders, atypia, and the majority are MDM2-amplified. Finally, pseudolipoma of Glisson capsule differs from lipoma by location (involves Glisson capsule rather than the hepatic parenchyma proper) and composition of predominantly necrotic lipocytes with frequent calcifications.
On a needle core biopsy, when the tissue sampled is entirely fatty, the diagnosis can be challenging. In the case of angiomyolipoma, an HMB-45 immunostain may prove useful to confirm the diagnosis, but may not be positive in all cases. If focal fatty change is suspected, correlation with imaging studies may be helpful, in addition to immunostains (S100 for adipocytes and HepPar-1 for hepatocytes).
3.3 Myelolipoma
3.3.1 Definition
Myelolipomas are rare tumors consisting of mature fat and bone marrow elements.
3.3.2 Clinical Features
Myelolipomas are most commonly encountered in the adrenal glands and rarely occur in extra-adrenal sites. Only a handful of cases have been reported in the liver [8–10]. Lesions are encountered in patients older than 40 years. Small tumors tend to be asymptomatic and are often incidental findings by radiologic studies. Large tumors may rupture and cause massive hemorrhage. Although adrenal myelolipomas may be associated with hormonally active neoplasms, such as adrenal cortical adenomas, pheochromocytoma, and Conn’s syndrome [11], no such associations have been described in hepatic myelolipomas. Of note, focal areas that are indistinguishable from myelolipomas can rarely be found in other hepatic tumors, such as angiomyolipomas [12].
3.3.3 Gross Findings
Most myelolipomas are small (<5 cm), although rare “giant myelolipoma” variants have been described outside the liver. Cut section shows a grey-red well-circumscribed nodule (Fig. 3.18).
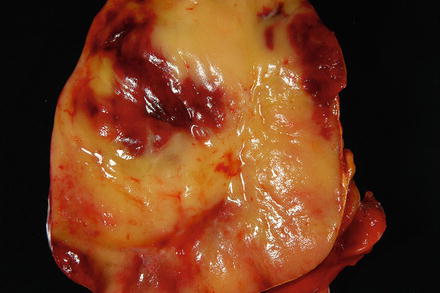

Fig. 3.18
Myelolipoma. The cut surface of the myelolipoma is heterogenous red-yellow and glistening
3.3.4 Microscopic Findings
Histologically, the lesion is circumscribed and non-encapsulated. It is composed of a variable mixture of mature adipocytes without atypia and hematopoietic elements representing all three lineages (erythrocytes, leukocytes, and megakaryocytes) (Fig. 3.19). The areas of fat may be irregularly fibrotic, but scattered throughout the fat there should be conspicuous islands of myeloid tissue. These islands are most readily identified by the presence of megakaryocytes, which appear large and multinucleated (Fig. 3.20). However, erythroid cells, particularly normoblasts, and myeloid cells should be easily found. Foamy macrophages may contain phagocytized fatty material within the tumor [9].
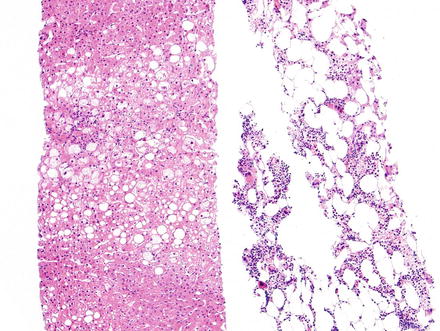
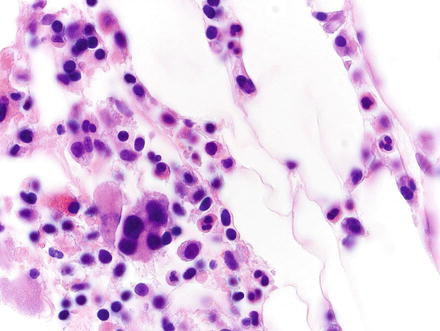

Fig. 3.19
Myelolipoma. Needle core biopsy of a liver mass shows slightly steatotic liver parenchyma on the left, and myelolipoma on the right composed of fat cells and islands of myeloid tissue

Fig. 3.20
Myelolipoma. Higher magnification of the previous case shows multinucleated megakaryocytes, nucleated red cells, and other hematopoietic elements
3.3.5 Immunohistochemical Features
Immunohistochemical stains are not required for diagnosis. On rare occasion, megakaryocytes might raise suspicion for metastatic carcinoma, and a negative keratin immunostain can be reassuring.
3.3.6 Differential Diagnosis
The differential diagnosis for hepatic myelolipoma includes extramedullary hematopoiesis, lipoma, and chloroma. Identification of both adipocytic cells and hematopoietic elements will exclude all three of these entities. For example, extramedullary hematopoiesis lacks a fatty component, lipoma lacks a hematopoietic component, and chloroma consists of only a single hematopoietic lineage—primitive myeloid cells. As with other multi-lineage lesions, sampling error in needle core biopsies can be a pitfall.
3.4 Leiomyoma
3.4.1 Definition
Primary hepatic leiomyoma is a benign smooth muscle tumor that occurs in the liver in the absence of a primary leiomyoma elsewhere [13].
3.4.2 Clinical Features
Primary hepatic leiomyomas are rare neoplasms with less than 30 cases reported in the literature [14]. They have been documented in both pediatric and adult populations (5–87 years), are most frequently found in adult women, and can be associated with Epstein-Barr virus (EBV) infection, particularly in the immunocompromised and post-transplant settings [14–20]. Some patients present with multifocal disease involving multiple organs: spleen, pancreas, lung, small and large intestine, and gallbladder. This leiomyomatosis is believed to result from multiple infectious events rather than metastatic disease [19]. Due to their low prevalence, the biological behavior of these EBV-induced neoplasms is unclear.
3.4.3 Gross Findings
These tumors range from 1 mm to 19 cm in diameter. On cut section, they are well-circumscribed, unencapsulated, and have a tan-white, whorled, fibrous cut surface.
3.4.4 Microscopic Findings
Most leiomyomas are highly cellular and consist of short fascicles of spindled well-differentiated smooth muscle cells. These fascicles intersect at perpendicular angles and the spindle cells are uniform, have abundant eosinophilic cytoplasm, and contain blunt-ended nuclei without cytologic atypia (Figs. 3.21, 3.22, and 3.23). Variable fibrosis, myxoid change, calcification, and degenerative changes may be seen. Lesions associated with EBV infection frequently display areas containing primitive round cells and prominent intratumoral T lymphocytes [19].
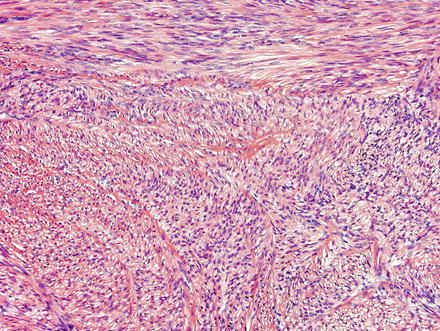
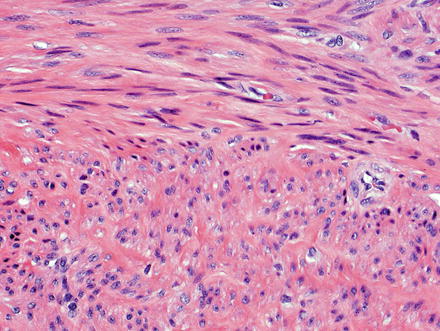
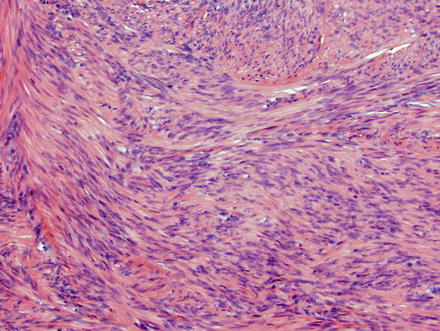

Fig. 3.21
Leiomyoma. These lesions appear similar to those seen elsewhere in the body, with intersecting fascicles of smooth muscle

Fig. 3.22
Leiomyoma. The nuclei are cigar-shaped with blunt ends, and the fascicles are arranged perpendicularly. In this example, one bundle of smooth muscle is cut longitudinally, while another is cut in cross-section

Fig. 3.23
Leiomyoma. When the nuclei palisade, one might consider a schwannoma or GIST; these lesions can be excluded with positive staining for smooth muscle actin stain and negative staining for S100, CD117, and DOG-1
3.4.5 Immunohistochemical Features
All leiomyomas express SMA and less frequently desmin. In tumors driven by Epstein-Barr virus, in situ hybridization for EBV-encoded mRNA (EBER) shows nuclear reactivity.
3.4.6 Differential Diagnosis
The differential diagnosis includes the malignant smooth muscle counterpart—leiomyosarcoma. Compared to the benign leiomyoma, leiomyosarcoma shows atypia, necrosis, and increased mitotic activity (>10 mitoses in 50 high-power fields). Immunohistochemistry will not be useful in this differential diagnosis, but can be especially helpful with the remaining differential diagnoses, which includes other cellular spindle cell lesions that can be found in the liver including: gastrointestinal stromal tumors, cellular schwannoma, and angiomyolipoma.
Gastrointestinal stromal tumors (GIST) are immunoreactive for CD34, CD117, and/or DOG-1, while leiomyomas are negative. Cellular schwannomas are encapsulated, diffusely express S100, and lack expression of smooth muscle markers. Angiomyolipoma can express SMA, but immunohistochemistry for HMB-45 and Melan-A is positive in angiomyolipoma while not in leiomyoma. Additionally, the cells in angiomyolipoma are typically nested and have clear or finely granular cytoplasm.
3.5 Benign Peripheral Nerve Sheath Tumors
3.5.1 Definition
Cells of the peripheral nerve sheath can give rise to an array of benign lesions such as schwannoma (derived from Schwann cells), neurofibroma (a mixture of Schwann cells, fibroblasts, perineurial-like cells, and residual nerves in a myxoid collagenous matrix), perineurioma (derived from perineurium), and granular cell tumor (thought to arise from Schwann cells).
3.5.2 Clinical Features
Schwannomas and neurofibromas represent the most common primary hepatic peripheral nerve sheath tumors. Hepatic perineuriomas are not reported, while granular cell tumors have been reported only in the extrahepatic biliary tree [21–26].
Schwannomas arise from Schwann cells in peripheral nerves. Primary hepatic schwannomas are highly associated with neurofibromatosis, although reports unassociated with the syndrome also exist [27]. Primary hepatic schwannomas are found in adult patients (35–74 years) with a female predilection [28]. Some are discovered incidentally, but the most common presenting symptom is epigastric pain or discomfort. Although many reported hepatic schwannomas occur in patients of Asian descent and with Hepatitis B infection, there has been no report of a causal relationship with viral hepatitis. Due to frequent cystic degeneration, these lesions are often mistaken on imaging studies for hydatid cysts, hepatobiliary cystadenoma, or cystic metastases [28, 29].
Neurofibromas and plexiform neurofibromas are highly associated with neurofibromatosis type 1 (NF1) and primary hepatic neurofibromas can be found in pediatric patients as young as age 4 [30]. The most common intra-abdominal areas affected are the duodenum and stomach, and hepatic involvement is very rare, with less than 20 cases reported in the literature. Clinical presentation includes pain and signs of intestinal or biliary obstruction [31–33], and patients often have plexiform neurofibromas elsewhere in the body at time of detection. Malignant transformation is a rare but serious complication. The lifetime risk of malignant transformation in a peripheral nerve sheath tumor is 7–13 % [34–36].
3.5.3 Gross Findings
Primary hepatic schwannoma of the liver is typically a well-demarcated lesion, surrounded by a true fibrous capsule. It ranges in size from 4 to 30 cm (mean 18.9 cm) [37]. Primary hepatic schwannomas are yellow-white in color and elastic-hard on cut section. Larger lesions often have areas of cystic degeneration.
Neurofibromas associated with neurofibromatosis type 1 are plexiform and involve the liver in an intrahepatic portal distribution. These lesions can extend into the gallbladder fossa and the pancreatic head, neck, and tail [30]. Neurofibromas are multinodular lesions involving multiple nerves or nerve branches, conferring a “bag of worms” appearance. The cut surface is grey-tan and the consistency ranges from fibrous to gelatinous. These lesions lack the degenerative hemorrhagic or cystic changes commonly seen in schwannomas.
3.5.4 Microscopic Findings
Histologically, primary hepatic schwannomas are the same as those found in other parts of the body. They are encapsulated tumors that arise within nerve sheaths and consist of a highly ordered cellular component with little stromal matrix (Antoni A area) and a hypocellular myxoid component with a loose meshwork of cells (Antoni B area) (Figs. 3.24, 3.25, and 3.26). The cells in both areas are uniform bland spindle-shaped cells with tapered nuclei. Focal palisading of these nuclei yields the characteristic Verocay bodies seen in schwannomas of any location (Fig. 3.27). Larger schwannomas have a tendency to undergo secondary degeneration and exhibit pseudocystic regression, hemorrhage, and calcification (Figs. 3.28, 3.29, 3.30, and 3.31). Similar to schwannomas found in other areas of the gastrointestinal tract, hepatic schwannomas are often surrounded by a rim of lymphoid cells (lymphoid cuff) [38].
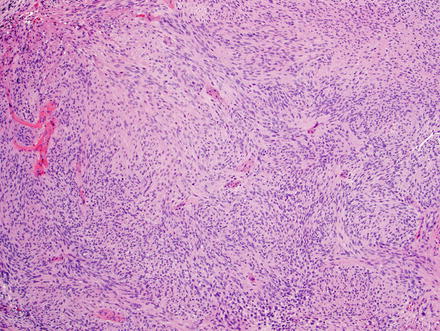
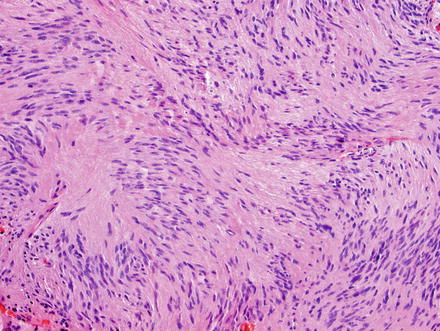
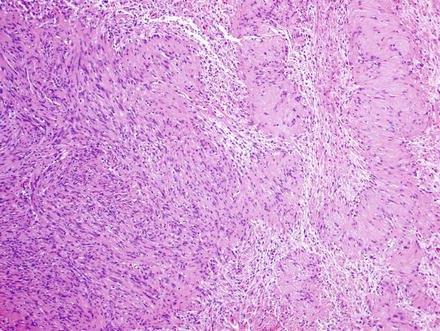
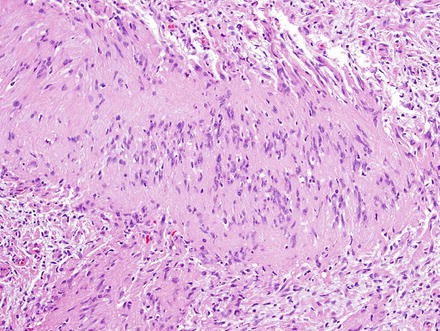
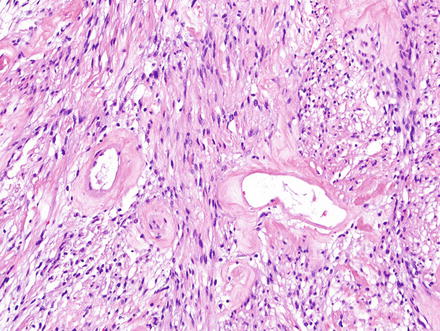
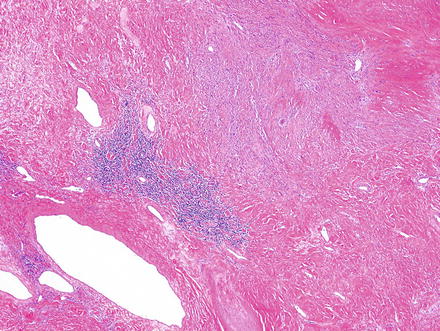
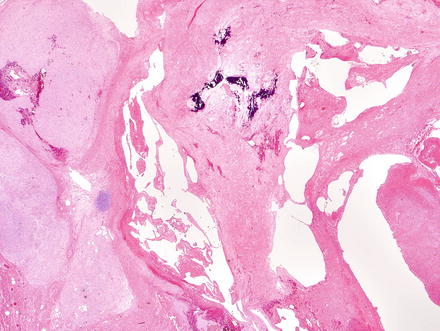
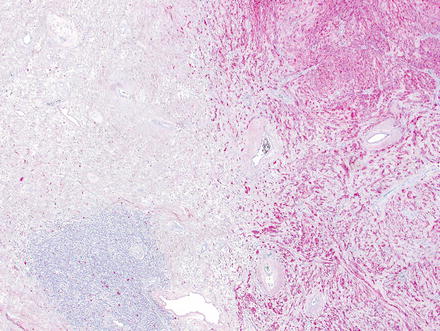

Fig. 3.24
Schwannoma. The lesion contains areas of high and low cellularity, best seen at low magnification

Fig. 3.25
Schwannoma. The spindle cells have fibrillary cytoplasm and tapered nuclei which characteristically palisade

Fig. 3.26
Schwannoma. There are densely cellular areas (Antoni A) to the left of this field, while less dense areas (Antoni B) are seen to the right

Fig. 3.27
Schwannoma. The palisaded areas create characteristic Verocay bodies

Fig. 3.28
Schwannoma. Hyalinized and wide-open vessels are characteristic

Fig. 3.29
Schwannoma. Areas of ancient change may appear hypocellular, contain only hyalinized vessels and collagen, and may show some lymphoid aggregates

Fig. 3.30
Schwannoma. This ancient schwannoma shows hyalinized vessels, low cellularity and calcification

Fig. 3.31
Schwannoma. An S100 immunohistochemical stain highlights the Schwann cells (right) in an area adjacent to ancient changes (left)
Hepatic neurofibromas also appear similar to those found in other parts of the body. They are composed of irregular interlacing fascicles of spindle cells with ovoid to spindled dark wavy nuclei (Figs. 3.32 and 3.33). The backdrop is composed of variable proportions of loose myxoid matrix and coarse collagen bundles which impart a “shredded carrots” appearance (Figs. 3.34 and 3.35). Mast cells, lymphocytes, and xanthoma cells may be scattered throughout the lesion. Atypia is not a feature of malignancy in the absence of increased cellularity, mitotic activity, and necrosis.
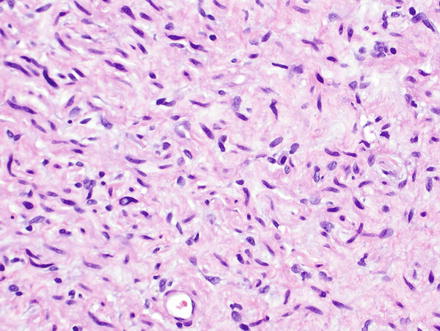
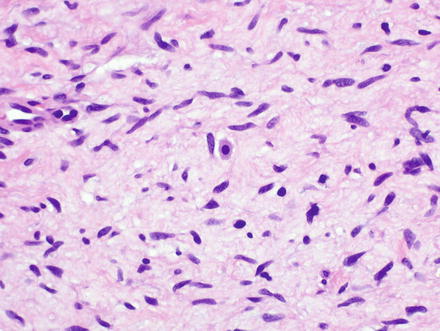
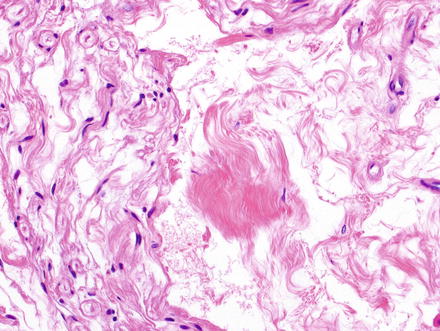
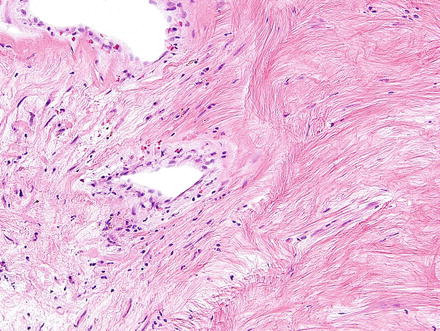

Fig. 3.32
Neurofibroma. Neurofibromas contain ovoid to wavy dark spindle cells in a backdrop of loose myxoid matrix and collagen

Fig. 3.33
Neurofibroma. Mast cells are frequently found in neurofibromas and have abundant granular cytoplasm with a centrally placed round nucleus, imparting a “fried egg” appearance (center of field)

Fig. 3.34
Neurofibroma. The characteristic dense fibrils of collagen are seen to the center and right of this field. These loose fibrils of collagen have been likened to “shredded carrot.” Contrast to this are the spindle cells at the left

Fig. 3.35
Neurofibroma. Neurofibromas may have paucicellular fibrous areas that are difficult to interpret. Immunostains are helpful in these cases
3.5.5 Immunohistochemical Features
Schwannomas are strongly and diffusely reactive for S-100 protein, desmin, and neuron-specific enolase (NSE). They are nonreactive for SMA, CD34, EMA, HHF-35, and GFAP [38].
Because neurofibromas contain a mixture of cells, an S-100 stain is positive only in a subset of spindle cells and is not as diffusely positive as it is in schwannomas. Neurofibromas can also express CD34, GFAP, and EMA (Fig. 3.36).
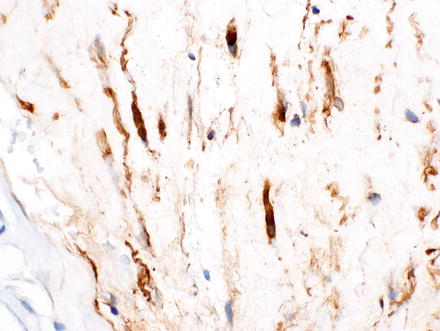

Fig. 3.36
Neurofibroma. A CD34 immunostain highlights the spindle cells in the previous case
3.5.6 Differential Diagnosis
When morphology is challenging, distinguishing schwannoma from neurofibroma can be achieved with immunohistochemical stains, as noted above. Ancient schwannomas can be paucicellular and resemble a hyalinized solitary fibrous tumor, but an S100 immunohistochemical stain can confirm nerve sheath differentiation.
3.6 Chondroma
3.6.1 Definition
A chondroma is a benign cartilagenous neoplasm.
3.6.2 Clinical Features
Only one case of a primary hepatic chondroma has been reported, occurring in an asymptomatic 44-year-old woman. After following the lesion radiographically for 6 years, the lesion was excised due to continuous growth [39].
3.6.3 Gross Findings
Chondromas are well-demarcated, spherical, or ovoid lesions ranging from 0.3 to 6.5 cm. The cut surface is rubbery or gelatinous.
3.6.4 Microscopic Findings
Histologically, chondromas are well-circumscribed, lobulated lesions comprised of mature hyaline cartilage (Fig. 3.37). Chondrocytes can be seen in lacunae and are arranged in small clusters (Figs. 3.38 and 3.39). Enlarged nuclei and moderate pleomorphism may be seen, but these lesions have a very low mitotic rate. Variable amounts of calcification can be seen in a lace-like granular stippled pattern and ossification is common.
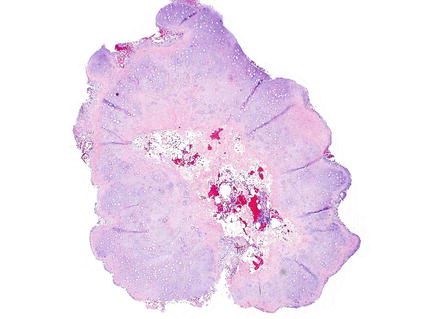
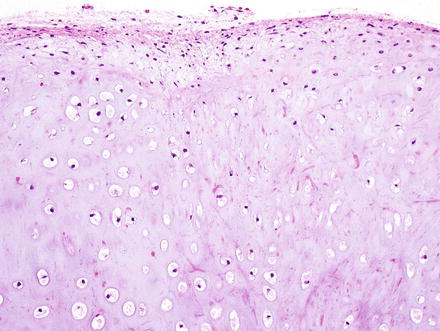
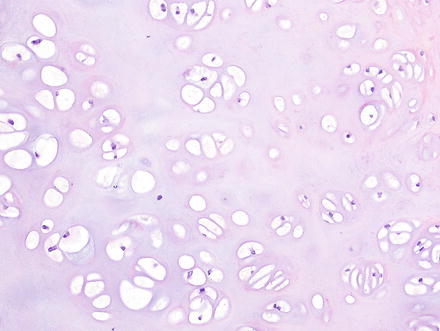

Fig. 3.37
Chondroma. At low power, chondromas are well-demarcated and lobulated lesions composed of cartilage

Fig. 3.38
Chondroma. The lesions have a well-circumscribed and unencapsulated border with variably dense clusters of chondrocytes

Fig. 3.39
Chondroma. The chondrocytes are arranged in clusters or cords and show no nuclear atypia or binucleation
3.6.5 Immunohistochemical Features
Immunohistochemistry is not a helpful tool for chondromas, and the diagnosis is made based on the distinctive chondroid morphology.
3.6.6 Differential Diagnosis
The differential diagnosis includes epithelioid hemangioendothelioma, extraskeletal myxoid chondrosarcoma, and metastatic chondrosarcoma. Epithelioid hemangioendotheliomas can have a prominent myxoid backdrop, but the presence of intracytoplasmic lumina, sometimes containing red blood cells, indicates the vascular nature of the neoplasm; CD31 and/or CD34 can confirm this. Extraskeletal myxoid chondrosarcoma can also have a prominent myxoid matrix and present as a lobulated mass. However, the cells have marked cytologic atypia and are arranged predominantly in cords. Features that can lead to misdiagnosis of metastatic chondrosarcoma include chondrocyte atypia, increased cellularity, and chondroblastoma-like features. Take care not to over-call these features and correlate with additional clinical and radiology information.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








