, Adele Taibbi1 and Massimo Midiri1
(1)
Department of Radiology, University Hospital, Palermo, Italy
2.1 Hepatic Cysts
A simple hepatic cyst is a single, unilocular lesion containing homogeneous serous fluid surrounded by a single layer of cuboidal epithelium, identical to that of bile ducts, and a thin underlying layer of fibrous stroma [1].
Usually, a correct diagnosis is already allowed by means of grayscale US thanks to a homogeneous anechoic appearance, a thin or imperceptible wall with posterior acoustic enhancement. Some internal thin septa may be present, but thick septa should suggest an abscess, parasitic infection, or cystic neoplasm. In particular, at CEUS, cystic tumors of biliary origin and cystic metastasis usually present a thick capsule, thick internal septa, or mural nodules which show contrast enhancement suggesting the presence of viable tissue [2].
Furthermore, hemorrhagic or complicated cysts may show hypoechoic inhomogeneous appearance instead of typical anechoic aspect on conventional US scan. In these cases, CEUS can depict the absence of vascularization throughout the vascular phase, suggesting cystic nature.
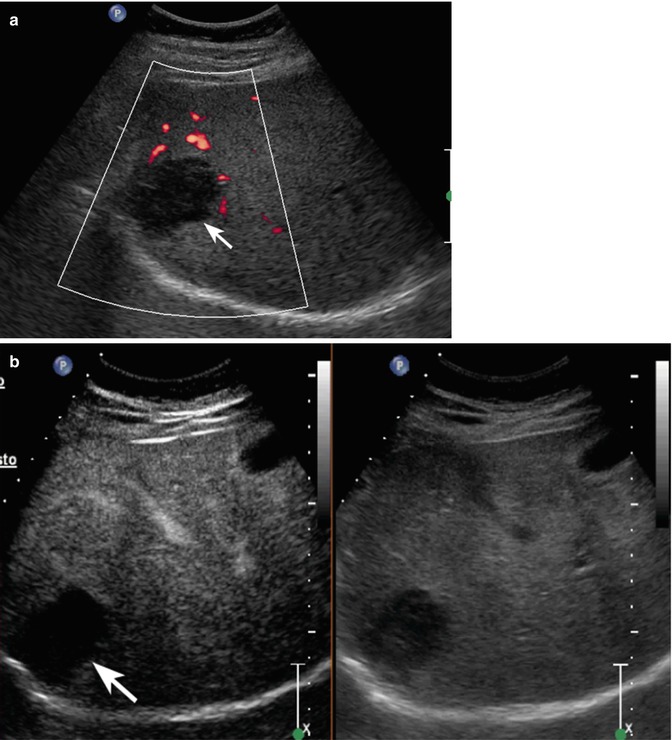
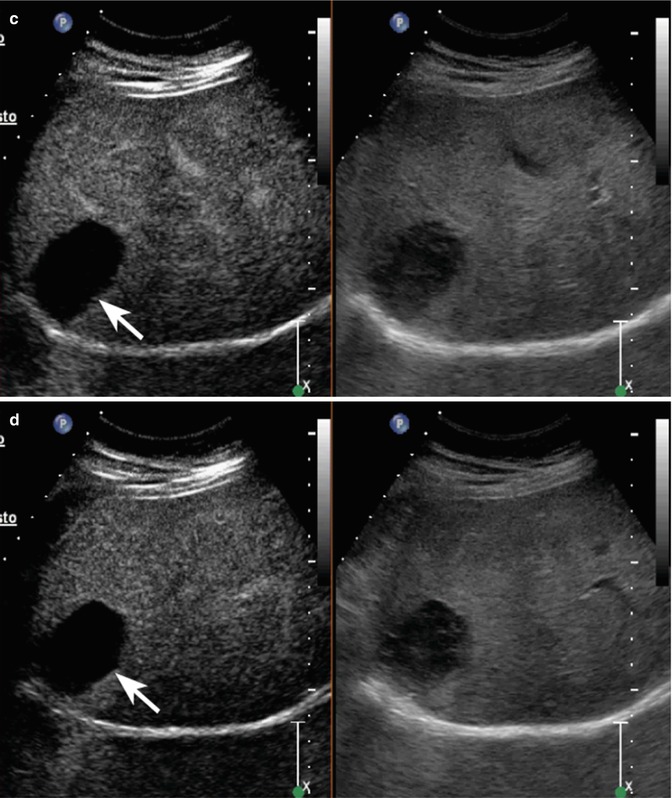


Fig. 2.1
Complicated cyst in a 48-year-old man. (a) Oblique ascending right subcostal baseline image shows a markedly hypoechoic lesion sized 3.5 cm in the subcapsular region of the VII hepatic segment not showing vascular signal at power-Doppler evaluation (arrow). (b–d) At CEUS, the lesion shows lack of contrast enhancement throughout the vascular phases (arrows)
2.2 Hydatid Cyst
Hydatid disease is a parasitic infestation by a tapeworm of the genus Echinococcus.
Cystic hydatid disease usually affects the liver (50–70 %) and less frequently the lung, the spleen, the kidney, the bones, and the brain. Liver hydatidosis can cause dissemination or anaphylaxis after a cyst ruptures into the peritoneum or biliary tract. Infection of the cyst can facilitate the development of liver abscesses and mechanic local complications, such as mass effect on bile ducts and vessels that can induce cholestasis, portal hypertension, and Budd-Chiari syndrome. At baseline US, hydatid cysts may present a solid aspect because of its inhomogeneous complex appearance. Parasitic cyst usually presents as a single, unilocular cyst or multiseptated cysts, showing “wheel-like,” “rosette-like,” or “honeycomb-like” appearances. “Snowstorm” sign may appear as multiple internal echogenic foci within the cyst cavity (hydatid sand). A correct diagnosis is of clinical relevance since biopsy of these lesions is not recommended in order to avoid severe adverse events. Usually, CEUS shows lack of enhancement of septa separating daughter cysts [3, 4].
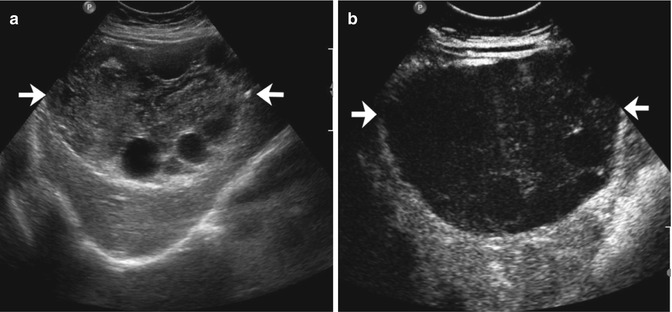

Fig. 2.2
Hydatid cyst with daughter cysts in a 62-year-old man. (a) At baseline US, a large heterogeneous hypoechoic mass sized 13.1 cm with multiple internal cystic areas is detected in the right hepatic lobe (arrows). (b) CEUS depicts the lesion as a constantly avascular mass (arrows)
2.3 Hemangioma
Hemangioma is the most frequent primary benign tumor of the liver found out with a prevalence range varying from 1 to 20 % and a higher incidence in females (women/men: 2/1–5/1).
At histopathological analysis, hemangioma is characterized by vascular lacunae lined by a single layer of endothelial cells associated with a variable rate of fibrous tissue [5, 6]. The differential diagnosis with other hepatic lesions has a pivotal clinical impact since it does not require any treatment.
At grayscale US, the hemangioma can present a “typical” aspect—hyperechoic lesion, variable in size with homogeneous or slightly inhomogeneous echotexture, well-defined margins with or without posterior wall shadowing—making easy a correct diagnosis especially in patients with no history of malignancy or chronic liver disease. Usually, color- and power-Doppler evaluation does not show any vascular signal within or at the periphery of the lesion because of very slow blood flows that distinguish it. Except for patients with cancer history or known chronic HBV-HCV-related liver disease, the detection of a lesion presenting these imaging findings does not require any further investigation.
Nevertheless, on grayscale US, hemangioma may show atypical features, such as an inhomogeneous internal echotexture. In particular, hemangiomas larger than 4–5 cm may present a markedly inhomogeneous aspect, because of thrombohemorrhagic episodes, cystic degeneration, fibrosis or hyalinization, and calcium deposit [7]. In these latter cases, CEUS represents a useful tool in order to more precisely characterize the lesion demonstrating, as shown at contrast-enhanced CT and MR, peculiar and specific contrast enhancement patterns [8–10]. In fact, CEUS allows the depiction of typical peripheral nodular enhancement in the arterial phase followed by a progressive centripetal fill-in in the extended portal-venous phase, which is considered diagnostic for hemangioma on contrast-enhanced CT and MR [11]. Incomplete fill-in in the extended portal-venous phase can depend either on the lesion size and the presence of thrombohemorrhagic phenomena or fibrosis [12]. In a smaller percentage of cases, it is possible to observe a peripheral rim enhancement in the arterial phase followed by a progressive centripetal complete or incomplete fill-in [13]. And finally, lesions smaller than 2 cm can show a rapid and homogeneous uptake of contrast medium in the arterial phase with sustained contrast enhancement in the remaining phases [14]. This feature is suggestive of capillary hemangioma. Capillary hemangioma could cause misinterpretation since other benign (focal nodular hyperplasia, hepatocellular adenoma) and malignant lesions (well-differentiated hepatocellular carcinoma) present this contrast enhancement behavior. Hence, clinical history is of pivotal relevance since if a lesion presenting these findings at CEUS is found out in a patient with chronic hepatitis, further investigations such as MR with hepatocellular-specific contrast medium are needed in order to rule out a well-differentiated hepatocellular carcinoma or a dysplastic nodule [15, 16].
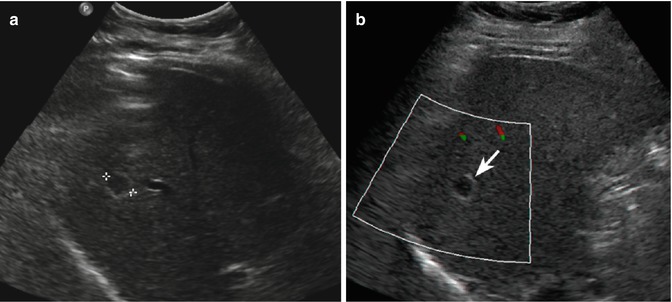
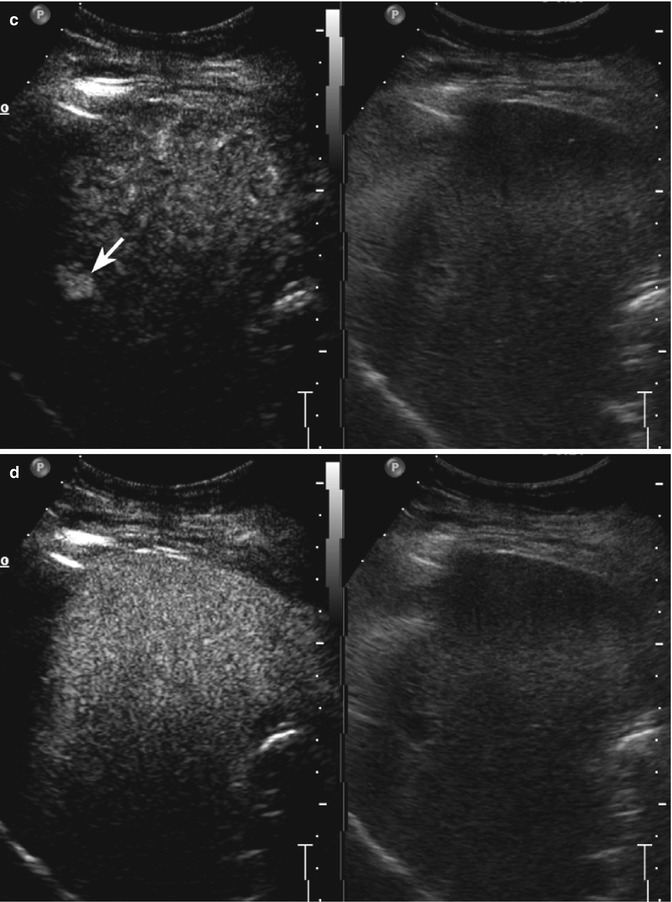
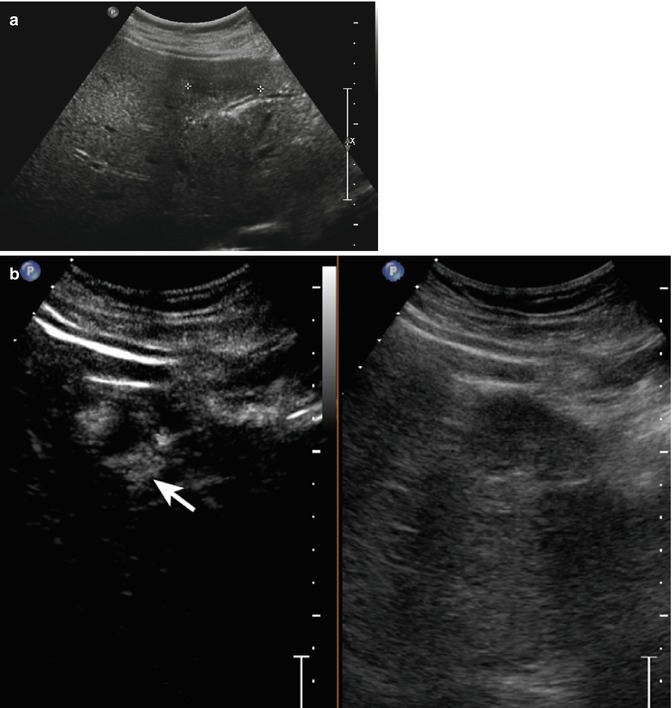
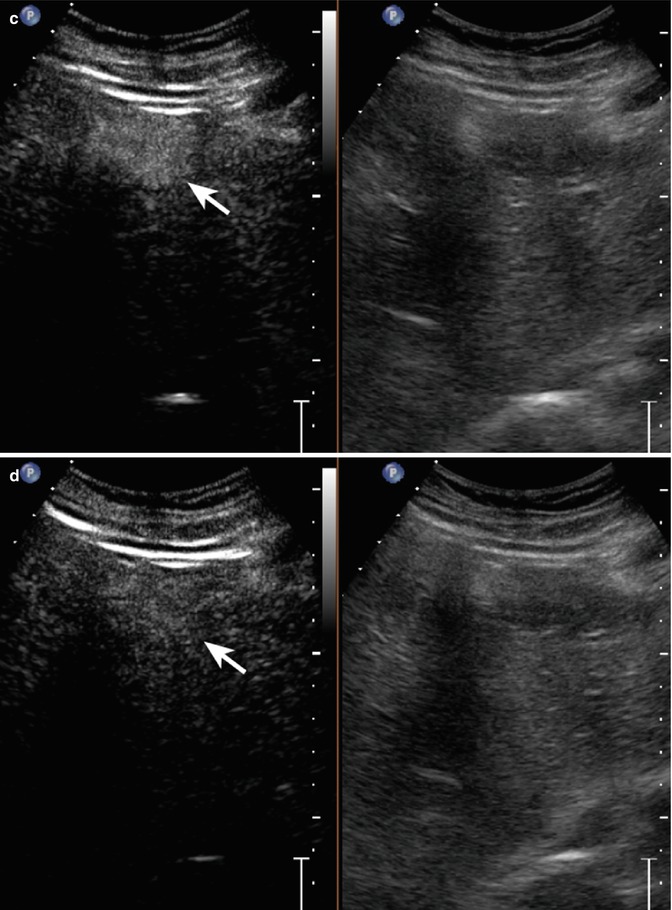
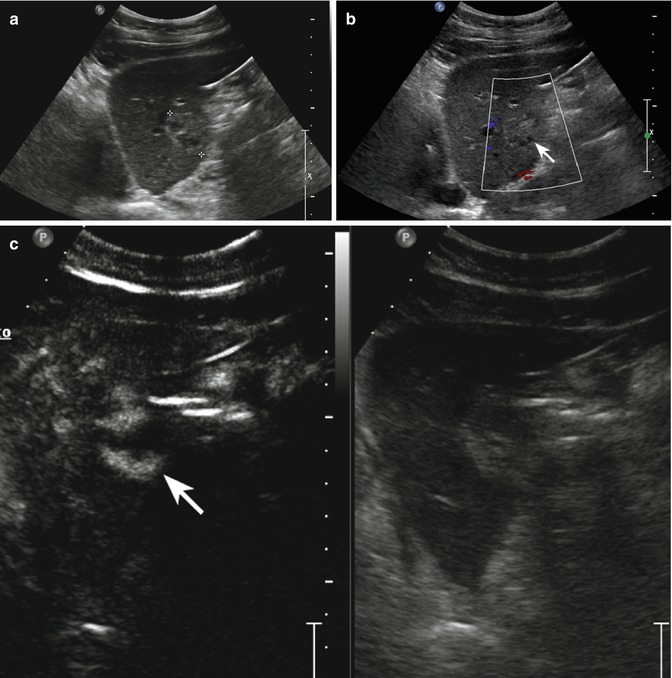
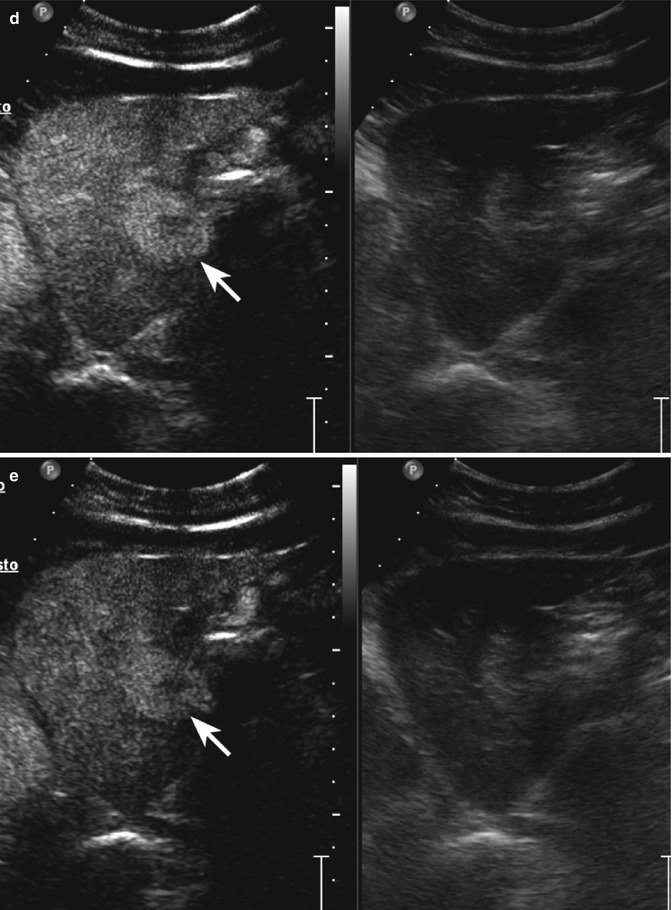
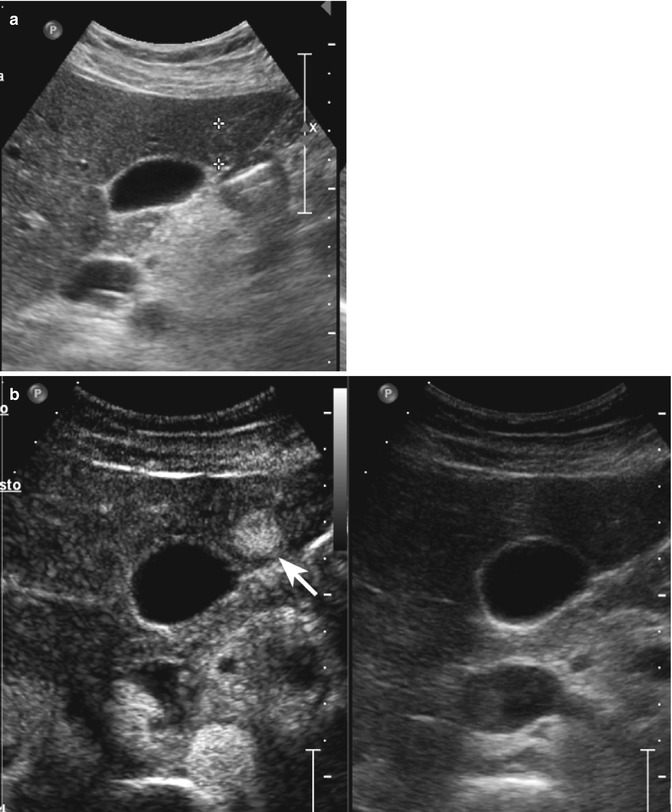
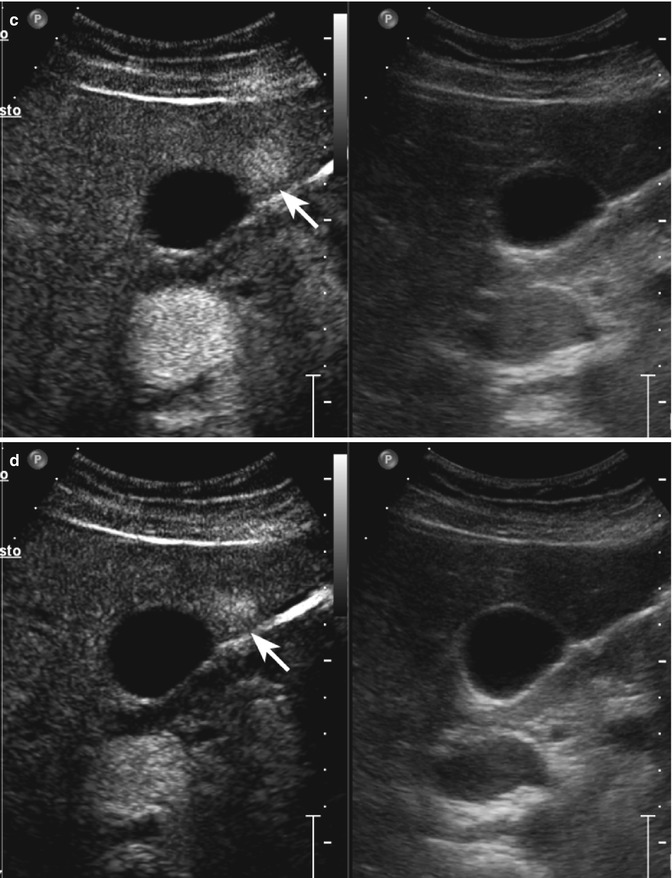
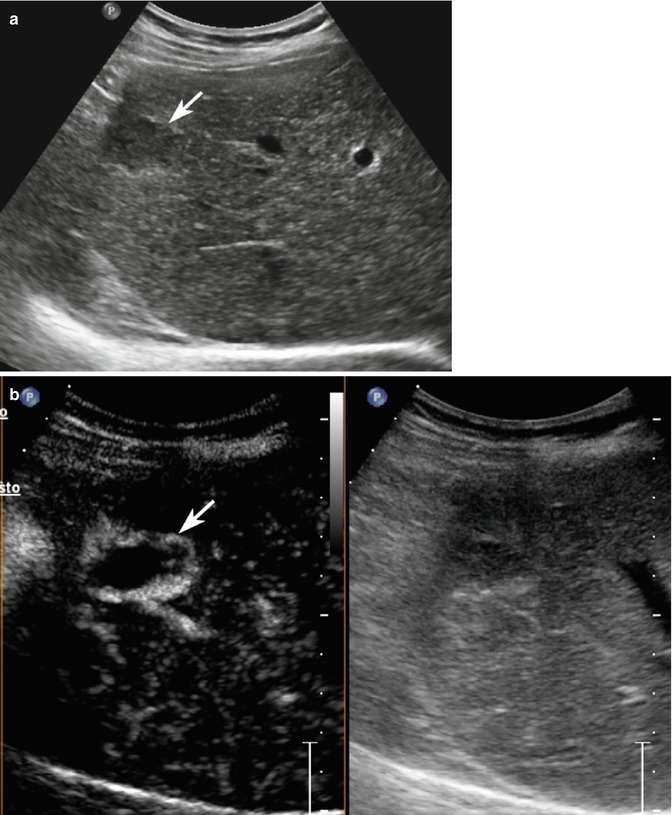
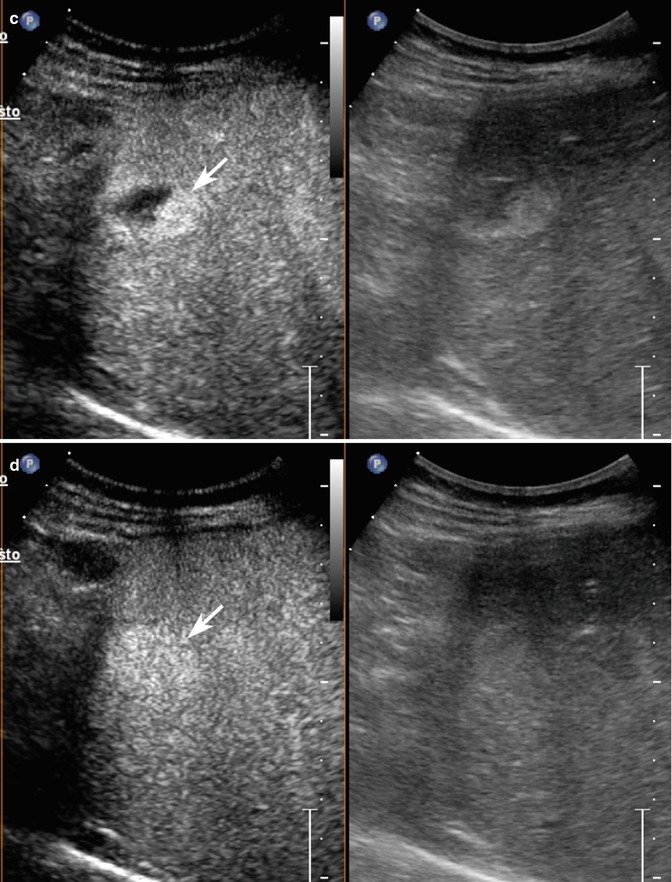
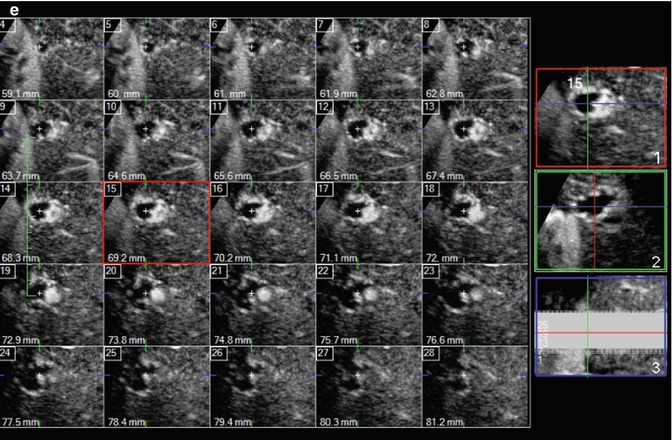
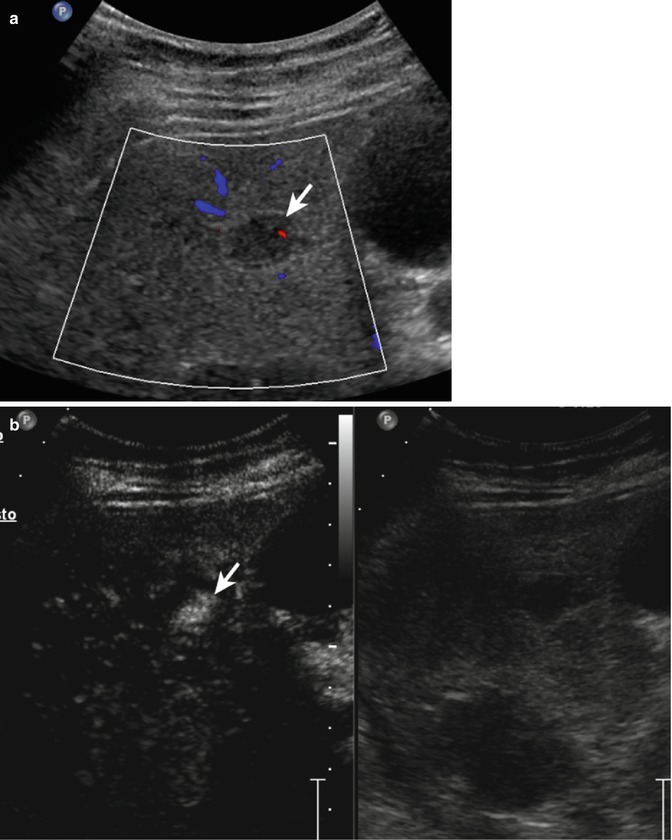
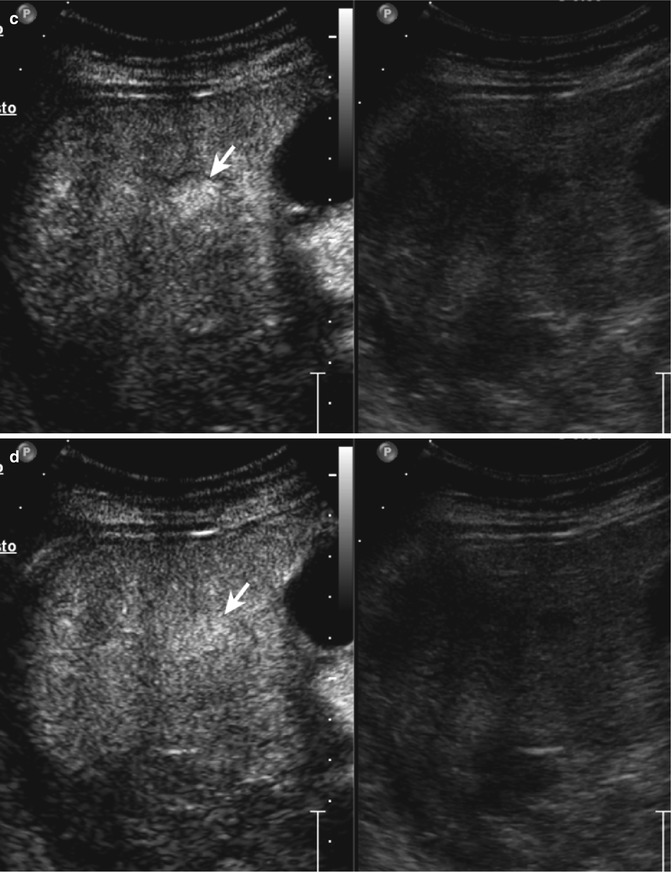
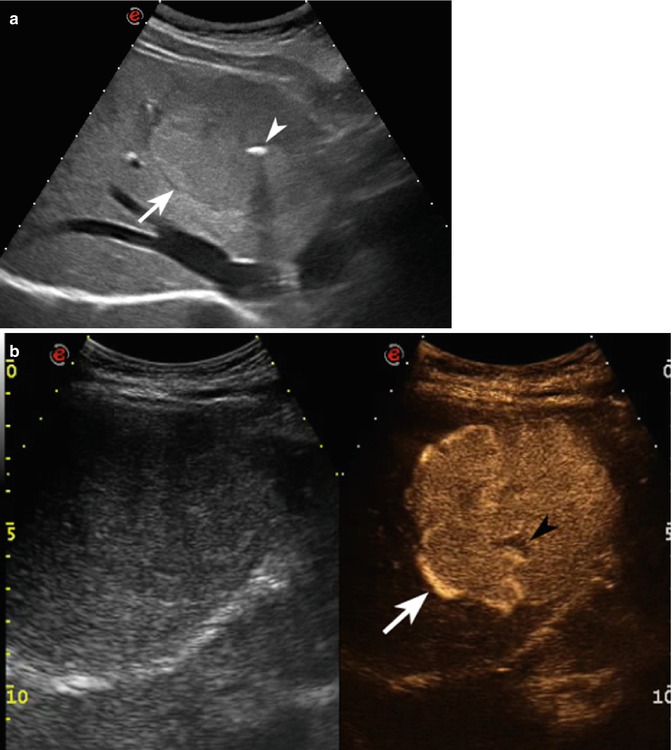
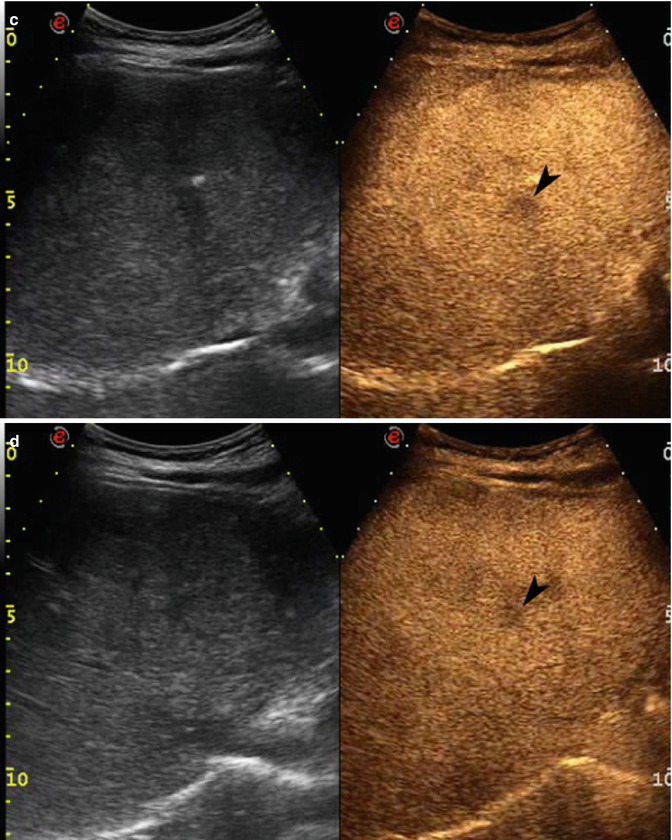
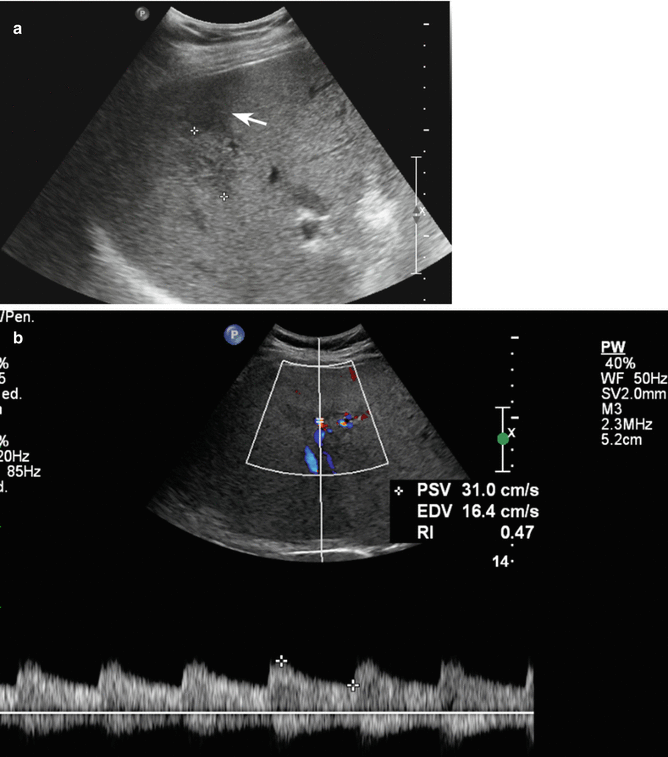
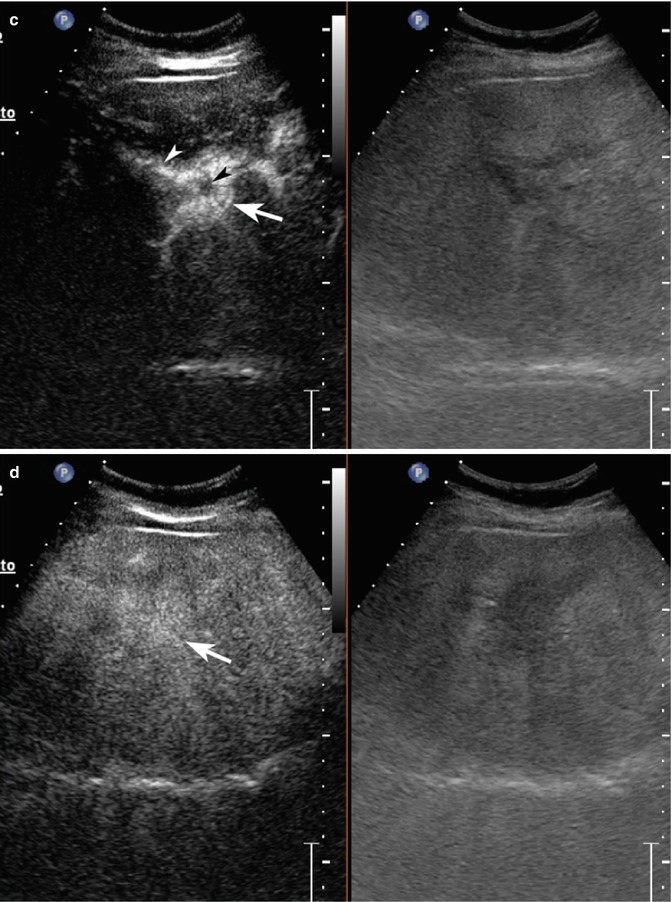
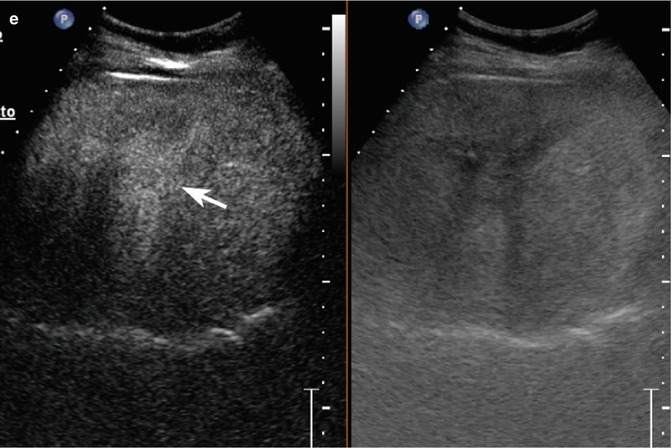
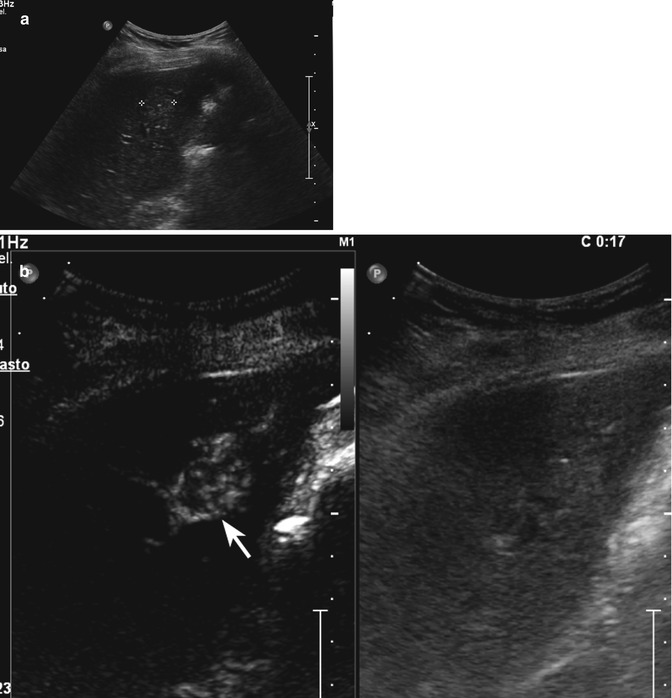
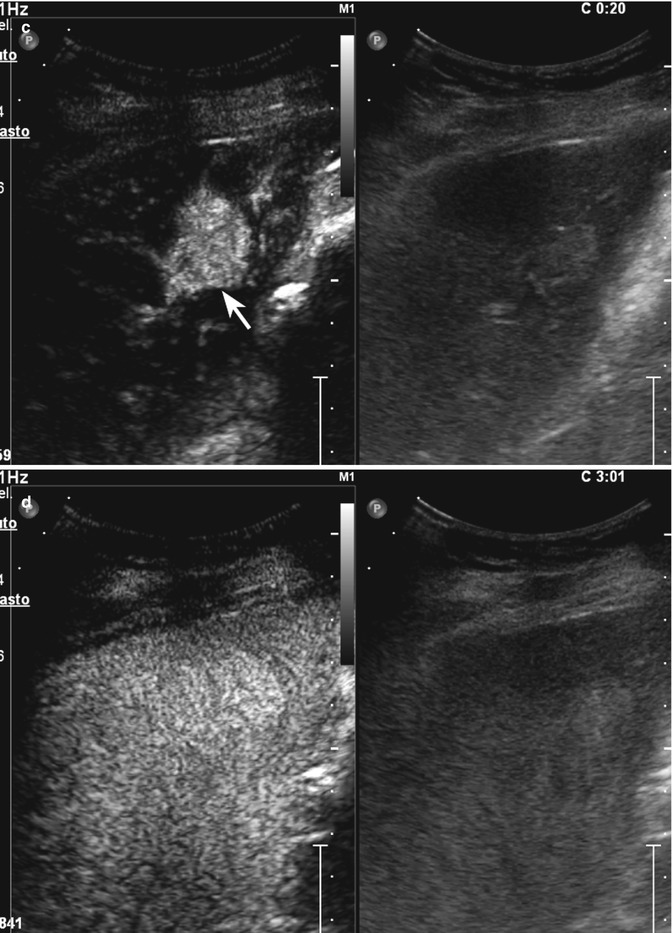


Fig. 2.3
Capillary hemangioma. (a) Baseline image shows an inhomogeneous lesion with hypoechoic central area surrounded by peripheral hyperechoic rim, sized 1.2 cm in the VII-VIII hepatic segment in a 52-years-old woman (calipers). (b) No vascular signal is evident at color-Doppler evaluation (arrow). (c) At CEUS in the arterial phase, the lesion presents a rapid and homogeneous uptake of contrast agent (arrow). The lesion is isovascular to the surrounding liver parenchyma in the late phase (d)


Fig. 2.4
Hemangioma in a 58-year-old man. (a) Baseline US image shows an homogeneous hypoechoic 3.6 cm-sized lesion, located in segment IV in the subcapsular region (calipers). (b) At CEUS, globular peripheral enhancement is appreciable in the arterial phase (arrow), followed by a progressive complete centripetal fill-in in the portal-venous phase (c). (d) The lesion is isoechoic with respect to the surrounding liver parenchyma in the late phase (arrow)


Fig. 2.5
Haemangioma in a 65-year-old woman. (a) Baseline US image shows a moderately inhomogeneous hyperechoic lesion, 2.9 cm in size, located in segment III in the subcapsular region (calipers). (b) No vascular signal is evident at color-Doppler evaluation (arrow). (c) At CEUS, globular peripheral enhancement is appreciable in the arterial phase (arrow), followed by a progressive centripetal fill-in in the portal-venous phase (d), incomplete in the late phase (e) (arrows)


Fig. 2.6
Capillary hemangioma in a 45-year-old man. (a) Baseline image shows an inhomogeneous moderately hyperechoic lesion sized 1.4 cm in the IV hepatic segment (calipers). (b) At CEUS in the arterial phase, the lesion shows a rapid and homogeneous uptake of contrast agent (arrow). The lesion reveals a sustained contrast enhancement remaining hyperechoic with respect to the surrounding liver parenchyma during the portal-venous (c) and late phases (d) (arrows)



Fig. 2.7
Hemangioma in a 54-year-old asymptomatic woman. (a) Baseline US shows a slightly hypoechoic homogeneous lesion, 2.2 cm in size, located in segment VI in the subcapsular region (arrow). (b) At CEUS, globular peripheral enhancement is appreciable in the arterial phase (arrow), followed by a progressive centripetal fill-in, complete in the remaining vascular phases (c, d) (arrows). (e) 3D i-Slice reconstruction shows globular peripheral enhancement in each slice too


Fig. 2.8
Inside-out hemangioma in a 40-year-old woman. (a) Oblique ascending right subcostal baseline image shows a homogeneous hypoechoic 2.2 cm-sized mass in fatty liver with tiny vascular signal at color-Doppler evaluation (arrow) in the V hepatic segment; (b) in the early arterial phase (20 s after SonoVue injection®), the lesion shows a central enhancing focus (arrow); (c, d) in the portal-venous and late phases, a progressive and complete centrifugal fill-in is shown (arrows)


Fig. 2.9
Focal nodular hyperplasia in a 31-year-old woman. (a) Baseline US image shows an isoechoic lesion sized 7.2 cm in the VIII hepatic segment (arrow) with small calcification in the central portion (arrowhead). (b) At CEUS, the lesion appears highly and homogeneously hypervascular in the arterial phase (arrow) showing a sustained contrast enhancement during the remaining portal-venous (c) and late (d) phases, except for a central constantly avascular area corresponding to the “central scar”(arrowheads)



Fig. 2.10
Focal nodular hyperplasia and focal fatty sparing. (a) Baseline US shows a 3.4-cm slightly hypoechoic lesion located in segment V (calipers) with intralesional arterial signal at pulsed-Doppler evaluation (b). Adjacent to the above mentioned lesion, a hypoechoic area with ill-defined margins is also appreciable in a (arrow). (c) At CEUS in the arterial phase, the lesion shows a clear-cut and homogeneous contrast enhancement (arrow) except for a small central hypoechoic area representing the central scar (black arrowhead). A feeding vessel is evident too (white arrowhead). The lesion presents sustained enhancement in the portal-venous (d) and late (e) phases (arrows). The area surrounding the FNH is not evident throughout the vascular study suggesting a pseudolesion (focal fatty sparing)


Fig. 2.11




Focal nodular hyperplasia in a 28-year-old woman. (a) Baseline US shows a 1.7-cm slightly hyperechoic lesion located in segment V (calipers). (b) CEUS depicts the spoke-wheel sign in the early arterial phase (arrow). (c) In the late arterial phase, the lesion shows a clear-cut and homogeneous contrast enhancement (arrow) (d) The lesion is isovascular with respect to the surrounding liver parenchyma in the late phase
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







