Fig. 1.1
Guinea pig urothelium stained for SNAP23 (green) and nuclei (Blue). Umbrella cells are strongly labeled. Lamina propria can be seen in faint green (background) (Avelino et al. 2015)
While the urothelium has been historically viewed primarily as a barrier, it is becoming increasingly appreciated as a responsive structure capable of detecting physiological and chemical stimuli and able to release a significant number of signaling molecules Thus, urothelial cells display a number of properties similar to sensory neurons and can use several signal-transduction mechanisms that perform an intricate cross talk with primary afferents located in the sub-urothelium. Urothelial cells express receptors for bradykinin, neurotrophins, as well as purinergic, cholinergic, adrenergic, and protease-activated receptors. Amiloride/mechanosensitive Na + channels and several transient receptor potential channels are also found in the urothelium (Table 1.1). In addition, urothelial cells can release neurotransmitters and signaling molecules such as nitric oxide, ATP, acetylcholine, prostaglandins, substance P, and neurotrophins (Table 1.1) that influence the excitability of suburothelial afferent nerves. Mediators released by urothelial cells may eventually act indirectly on suburothelial myofibroblasts (also referred to as “interstitial cells”) that lie in close proximity to afferent nerves. Thus, it is believed that urothelial cells and myofibroblasts can participate in sensory mechanisms in the urinary tract in an autocrine/paracrine way.
Table 1.1
Receptors and signaling molecules present in the urinary bladder
Receptor/channel/signaling molecule | Location | Neurotransmitter/agonist/stimulus |
|---|---|---|
Receptors | ||
Cholinergic muscarinic (M2, M3) | D, U, S | Acetylcholine, muscarine |
Cholinergic nicotinic | U, S | Acetylcholine, nicotine |
Adrenergic alpha | U, D, S | Norepinephrine; epinephrine |
Adrenergic beta | U, D, S | Norepinephrine; epinephrine |
Degenerin/ENaC | U, S | Amiloride, mechanical |
Neurotrophins (trkA; p75; trkB) | U, D, S | NGF, BDNF |
U, D, S | Bradykinin | |
U, D, S | Substance P, neurokinin A | |
Estrogen receptor | U, D, S | Estradiol |
Proteinase-activated receptor | U, D, S | Thrombin; trypsin |
TRPV1 | U, S | Heat (43 °C), low pH, anandamide, vanilloids |
TRPV2 | U, S | Noxious heat (53 °C) |
TRPV4 | U, S | Cell stretch, moderate heat (24 °C), 4 alpha-PDD |
TRPM8 | U, S | Cold (8–28 °C), menthol, icilin |
TRPA1 | U, S | Cinnamaldehyde, mechanical |
P2X | U, D, S | ATP |
P2Y | U, D | ATP; UTP; ADP |
P1 | U, D | Adenosin |
U, D, S | Anandamide; cannabinoids | |
VEGF receptor | U, D, S | Vascular endothelial growth factor |
Examples of this phenomenon are well documented: distension of the bladder causes ATP release from urothelial cells that consequently stimulates P2 × 2/P2 × 3 receptors in suburothelial sensory; stimulation of urothelial cells with the vanilloid receptor agonists capsaicin and RTX increases their intracellular calcium and evokes transmitter (nitric oxide and ATP) release. It is noteworthy to mention that in aged mice, increased levels of ATP are released in the bladder lumen, resulting in increased primary afferent activity, suggesting that aging results in aberrant urothelial function.
Interstitial Cells
Interstitial cells of Cajal, ICC-like cells, or myofibroblasts found in the urinary bladder resemble the interstitial cells of Cajal (ICC) of the gastrointestinal tract. After some debate, there is some consensus that these cells should be referred as interstitial cells of Cajal (ICC) or just as interstitial cells (IC).
Based on their location within the bladder wall, several subgroups of IC have been identified. ICC of the lamina propria are stellate-shaped cells that have been found to be extensively linked by gap junctions. Detrusor IC are elongated non-networked cells arranged in circular, longitudinal, and oblique orientation, on the boundary of smooth muscle bundles; see Fig. 1.2. They make close structural connections with nerves, but the origin of the nerve fibers (afferent or efferent?) was never determined.
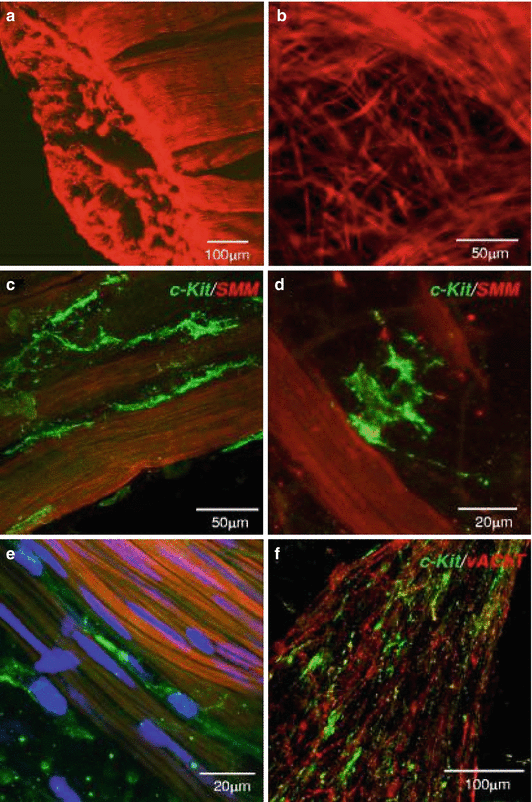

Fig. 1.2
Human detrusor ICC. (a) human detrusor labeled with anti-SM myosin (SMM). (b) At mucosa-detrusor interface, SMC were arranged in loose network, which was organized into distinct bundles in detrusor muscularis. (c–e) Representative human detrusor colabeled with anti-SM myosin and anti-KIT (green areas). Elongated, branched KIT-positive ICC track SM bundles and occupy spaces between bundles with obvious spiky morphology. (e) Nuclei counterstained with 4,6-diamidino-2-phenylindole (blue areas). (f) Detrusor ICC (green areas) associated with vAChT nerves (red areas) (From Johnston et al. J Urol. 2010;184:370–7)
Ultrastructurally, IC of the lamina propria present the characteristics of the classical gut myofibroblasts. These include bundles of fine cytoplasmic filaments, dense bodies, linear arrays of subsurface vacuoles, and the presence of an interrupted basal lamina. In the detrusor, electron microscopy studies showed that interstitial cells have flattened, dendritic-like processes, forming a branching network around individual muscle fascicles. In the cell soma and cytoplasmic processes, mitochondria, rough endoplasmic reticulum, and Golgi apparatus were observed, as well as intermediate filaments. Gap junctions and close appositions were described, but not specialized contacts with smooth muscle.
IC can be detected by immunostaining for the intermediate filament, vimentin. A subpopulation of these cells, in addition, also expresses the tyrosine kinase receptor, KIT. Some data is available regarding the functional role of IC in overactive bladders, but the emerging picture is still not clear. Some reports showed that overactive human bladder tissues had upregulated numbers of IC and enhanced contractility, which was more sensitive to a tyrosine kinase inhibitor. On the other hand, C-kit immunoreactivity was similar in patients with neurogenic bladders and controls and remained unchanged after treatment.
IC form an heterogeneous population, with some cells responding to cholinergic stimulation (detrusor IC) whereas others (lamina propria IC) do not. In contrast, they respond to application of ATP with calcium inflow. In this way, they appear to be ideally placed to respond to ATP released from urothelial cells, providing a putative transduction signaling system between the suburothelial sensory fibers and the detrusor muscle. In addition, they may eventually contribute to myogenic activity (nerve-dependent or spontaneous) due to their syncytial-like network.
Detrusor Muscle
The smooth muscle cells of the detrusor muscle are not clearly arranged in distinct layers, but run in all directions, forming groups of small functional units, or fascicles. Here, individual cells are evenly separated by an approximately 200 nm thick space containing collagen fibrils attached to a basal lamina. Specialized junctions, like zonulae adherens, and areas of sarcolemma apposition can be found in contiguous cells. Connexin-rich gap junctions were described in normal detrusor human bladder biopsies. Experimental studies suggest that gap junctions may have an important role in detrusor function. In animals with bladder outlet obstruction (BOO) leading to hypocontractility of the detrusor, connexin expression and the number of gap junctions were significantly reduced.
Innervation of the detrusor can be seen coursing the interstitium as Schwann cell-ensheathed axon bundles. Many axons form varicosities closely apposed to the muscle cells, forming neuroeffector junctions. Ultrastructural studies have shown that many of the varicosities are probably autonomic motor efferents, while others are undoubtedly primary afferents (Fig. 1.3).
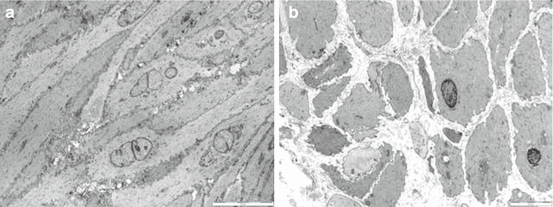

Fig. 1.3
(a) Normal detrusor. Normal detrusor myocytes are spindle-shaped mononuclear cells evenly aligned with minimal interstitial collagen and elastin in fascicles. (b) Ultrastructural features that correlated with each other and with poor postoperative voiding outcome were loss of fascicle arrangement, variable cell size, collagen in fascicles, and abnormal cell shape (From Blatt et al. J Urol. 2012;188:2294–9)
Several bladder dysfunctions show ultrastructural changes in the detrusor. These are particularly evident in muscle cells and in the interstitium (Fig. 1.4). In overactive human bladders, a well-described disjunction pattern was recognized, consisting of widened spaces between cells, reduction of intermediate cell junctions, and increase of protrusion junctions and ultraclose cell abutments. In a recent study, the detrusor muscle of patients suffering from bladder underactivity subsequent to bladder outlet obstruction also showed morphological abnormalities. These consisted of variations in muscle cell size and shape, abnormal fascicle arrangement, and collagenosis, loosely corresponding to the myohypertrophy pattern originally described by.
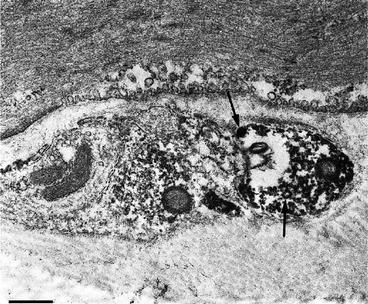





Fig. 1.4
Ultrastructural visualization of VR1 (presently know as TRPV1) immunoreactivity in nerve profiles found in the muscular layer. Immunoreactive varicosities lie in shallow grooves in the surface of smooth muscle cells. Small clear and large dense-core vesicles (arrows) can be seen (Adapted from Avelino et al. Neuroscience. 2002;109:787–98)
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








