Fig. 2.1
Conventional enteroclysis versus MR enterography. Conventional enteroclysis (a) shows small nodular defects (arrows) corresponding to pseudopolyps shown by transverse steady-state MR enterography sequence (arrows in b) in a patient with Crohn’s disease
Video capsule endoscopy (CE), introduced in 2000, has further contributed to the reduction in the use of barium examinations allowing the direct evaluation of the SB wall with a higher accuracy in the detection of early and superficial lesions [9–11].
The availability of these new radiological methods with a resulting reduction in the demand for barium studies has also made radiologists progressively less interested and able to perform these types of examinations [2].
Moreover, although these new imaging methods are less accurate than traditional radiology in the assessment of mural lesions, they allow evaluation of the bowel wall, its relationship with the parenchymal organs, peritoneal recesses, and the surrounding tissues. Recent literature shows a gradual replacement of the barium studies with new radiological methods (CE or CT or MR enterography) in most clinical situations.
Currently, a cross-sectional imaging study is the examination of choice for the evaluation of an SB neoplasm as it allows, with a single study, detection of a mass, its extraparietal extension, and the presence of pathologic lymph nodes or distant metastases [12].
Currently, according to new guidelines, CE and/or MR enterography have also supplanted barium studies in the screening of patients with familial adenomatous polyposis [13] and in other important clinical situations, such as suspected high small bowel obstruction (SBO) [14] and active obscure GI bleeding [15] in which MDCT, endoscopy, and CE are considered more accurate. In fact, a noninvasive MDCT scan allows evaluation of the obstruction or bleeding in terms of location, cause, and severity, with the application of multiplanar reconstruction images [14, 15].
Finally, the clinical and diagnostic approach to the patient with abdominal pain has significantly changed with ultrasound (US)—almost always the initial examination in a wide range of situations.
However, barium examinations, such as SBFT, are still the most common and accessible radiological methods for studying the small bowel [10], and their application is important especially in situations where other new techniques are limited. As such, these procedures can provide the correct diagnosis at a lower cost and with fewer complications (e.g., those due to sedation required for endoscopy) [2]. Moreover, they are often used as a second-line test to confirm CT, MR, or US findings and are considered the diagnostic study of choice to exclude the presence of a bowel stenosis before performing a CE.
Barium Imaging in Intestinal Malrotation
Intestinal malrotation, which is defined as a congenital abnormal position of the gut within the peritoneal cavity, may lead to potentially fatal sequelae, such as midgut volvulus. The midgut rotation process can be divided into three stages. In stage I, from the 5th to the 10th week of gestation, the midgut elongates forming the primary intestinal loop, which undergoes a physiological herniation into the umbilical coelom where it grows and rotates 90° counterclockwise around the axis of the superior mesenteric artery (SMA). Failure to return to the abdomen during stage I results in the development of an omphalocele. In stage II, during the 10th to the 12th week of gestation, the midgut retracts into the abdominal cavity. The small intestine returns first completing its final 90-degree counterclockwise rotation and thereby passing posterior to the SMA. The large intestine enters later making an additional 180-degree counterclockwise rotation resulting in the normal configuration of the colon. In conclusion, the normal whole rotation of the intestine is 270° in a counterclockwise direction. Rotational defects during this process can result in a number of abnormal conditions. Nonrotation of the midgut, also called left-sided colon, is the most common rotation anomaly that occurs if the primary intestinal loop fails to undergo the second rotation of 180°. The result is an aberrant positioning of the colon and cecum on the left side of the abdominal cavity with the small intestinal loops situated in the right side. The duodenum has its first and second parts normally situated but the third and fourth parts slope down to the right of the SMA. This condition can lead to midgut volvulus and duodenal obstruction. The reversed rotation, instead, arises when the primary intestinal loop undergoes an incorrect 180-degree clockwise rotation after the first 90-degree counterclockwise rotation resulting in a total 90-degree clockwise rotation with an abnormal position of the transverse colon in the retroperitoneal space, posterior to the duodenum and the SMA. Finally, mixed rotations of the midgut (also called malrotations) can occur, due to an uncoordinated rotation of the duodenum and the colon with one malrotated segment and the other partially or nonrotated [16]. The most frequent type of malrotation arises when the cephalic limb and the caudal limb of the primary intestinal loop undergo only the initial 90-degree rotation and the later 180-degree rotation, respectively. The result is a correct positioning of the distal end of the duodenum and a cecal position just inferior to the gastric pylorus, near the midline in a subhepatic or central position. This causes an increased risk of intestinal obstruction as a consequence of SB compression, especially the duodenum.
In stage III, from the 12th week up until the term of gestation, the mesentery of some intestinal segments becomes fixed to the posterior abdominal wall, whereas some others disappear. Initially, following rotation, the duodenum and pancreas are situated in the right upper quadrant. There, due to the pressure of the colon, they are compressed against the posterior abdominal wall with which their peritoneums fuse, disappearing, and the resulting retroperitoneal position of most of the duodenum and head of the pancreas. In addition, the mesentery of the ascending and descending colon fuses with the peritoneum of the posterior abdominal wall with both the ascending, except for about 3 cm of its caudal portion, and descending colon becoming retroperitoneal.
The mesenteries of the appendix, lower end of the cecum, and sigmoid colon remain free.
What undergoes major changes is the mesentery of the primary intestinal loop, also called the mesentery proper, which, at the end of the process, in case of normal rotation and fixation, extends from the duodenojejunal junction (ligament of Treitz) in the left upper quadrant to the ileocecal junction in the right iliac fossa.
Also in this stage, some different abnormalities may occur due to altered fixation processes.
Firstly an incomplete or, in extreme cases, a total lack of fixation of the ascending colon to the posterior abdominal wall can lead to an abnormal motility of the cecum alone (mobile cecum) or of both the cecum and other colonic segments, respectively, with an increased risk of volvulus. A malfixation of the mesentery proper may result in a reduced distance between the ligament of Treitz and the ileocecal junction, resulting in loose loops of bowel hanging on an unsteady and narrow pedicle that is prone to twisting, volvulus, and strangulation.
Furthermore, an incomplete fusion of the mesentery with the posterior wall of the colon can lead to the formation of retrocolic recesses, most frequently posterior to the ascending colon, with the risk of herniation and entrapment of SB loops.
Finally, if the cecum fails to descend to its normal position in the right iliac fossa, it can develop an abnormal fixation in the right upper quadrant with dense fibrous bands (Ladd’s bands) extending from the cecum to the retroperitoneum across the duodenum (Fig. 2.2) or, less frequently, between the colon and the duodenum (Fig. 2.3). The major risk is a variable degree of compression until complete obstruction of the duodenal loop.
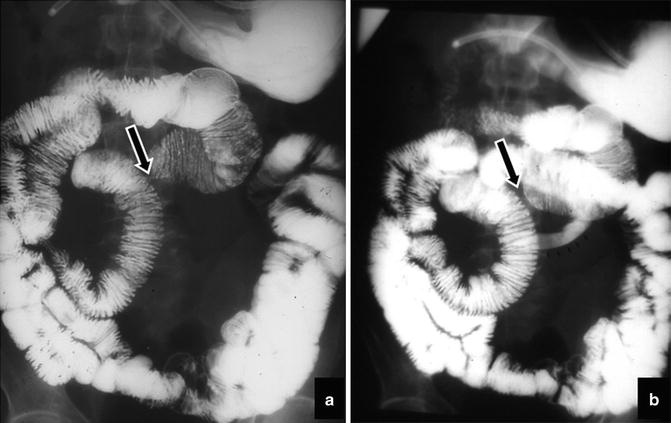
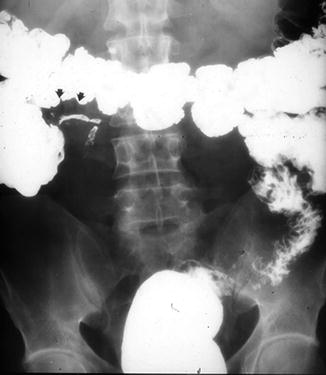

Fig. 2.2
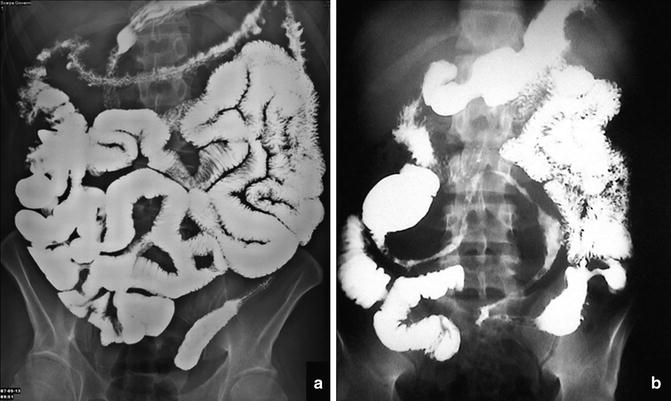
Malrotation with duodenal bands. Radiographs show an abnormal location of the duodenojejunal junction (arrow) and the proximal part of the jejunum in the right upper abdominal quadrant and the ileum in the left abdominal quadrants. In (b) the small black arrow shows narrowing of a jejunal loop due to adhesion (Ladd’s bands)

Fig. 2.3
Ladd’s bands. Radiograph shows the cecum fixed in the right upper quadrant due to adhesions (Ladd’s bands), histologically confirmed
Of all cases of malrotation, 60–80 % present in the first month of life, mostly in the first week. The most common symptoms of midgut volvulus are a generalized discomfort of the infant and bilious vomiting that needs an immediate investigation even in the absence of acute abdominal pain [17]. Similarly, a malrotation/volvulus must be suspected in any case of acute abdomen, even if bilious vomiting is absent.
However, the clinical presentation can be very variable and ambiguous, including both acute symptoms such as diarrhea, non-bilious vomiting, suspected infection or sepsis, shock or GI bleeding, and long-standing conditions of malabsorption and growth defects.
In the diagnosis of malrotation, the plain X-ray is rarely sufficient, except in cases of obvious complete duodenal occlusion, and an upper GI imaging is the preferred diagnostic modality, especially with the use of water-soluble contrast medium. Ionic hypertonic solutions, such as gastrografin, are to be avoided as aspiration causes pulmonary edema that may be fatal. A nasogastric tube is required to administrate oral contrast medium, because the duodenum may be obscured by a contrast-filled and distended stomach. Upper GI malrotation can present with some pathognomonic signs that include an abnormally positioned duodenojejunal junction (Fig. 2.4), a spiral, “corkscrew” or Z-shaped configuration of the distal duodenum and proximal jejunum, and a right located proximal jejunum (Fig. 2.5). The duodenojejunal junction is usually situated in the retroperitoneal cavity and to the left of the spine at the same level or higher than the duodenal bulb. In an anatomical variant, the junction can be displaced inferiorly due to the relative mobility of the ligament of Treitz, and can mimic a malrotation [18]. There are some other conditions that can mimic malrotations on upper GI exams, especially for the duodenum (a mobile, wandering, or inversum duodenum) and peritoneal ligaments. In case of acute intestinal obstruction, especially in children, upper GI images can show some pathognomonic findings such as a narrowed bowel, partially or completely occluded, with consequent dilatation of the proximal duodenum and a characteristic spiral or “corkscrew configuration” of the twisted distal duodenum.
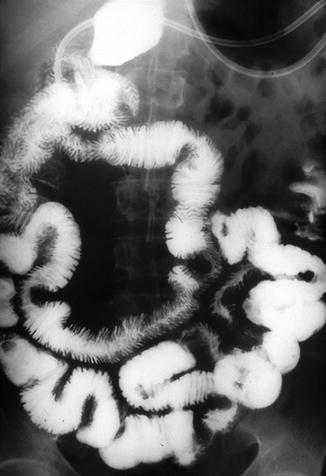
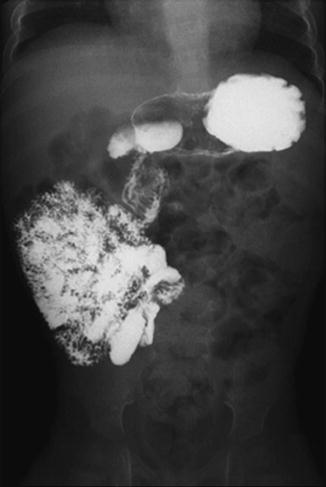

Fig. 2.4
Intestinal malrotation. Enteroclysis shows that the duodenum and jejunum are to the right of the spine

Fig. 2.5
Intestinal malrotation. Delayed radiograph shows the location of jejunal loops in the upper right side of the abdomen
When the obstruction is complete, the typical “corkscrew” image may not be seen because contrast medium may not enter the volvulized loops.
If the obstruction is caused by peritoneal bands, the duodenum can assume also a Z-shaped configuration [18].
The sensitivity of the upper GI series for the diagnosis of malrotation approximates 93 % [19] to 100 % [20]. However, in a retrospective study of 72 patients a significant number of false-positive upper GI diagnoses, supported by subsequent negative laparotomy, were observed. Particularly, 13 patients (18 %) were incorrectly diagnosed. In fact, after laparotomy, 6 (8.3 %) had normal anatomy, 3 (4.2 %) did not have a volvulus, and 4 (5.5 %) had a malrotation without a volvulus [21].
The diagnostic accuracy of upper GI in diagnosing malrotation may be improved by various maneuvers [18]. First of all, the correct position of the duodenojejunal junction should be detected at the beginning of the exam with the first bolus and studied in both the frontal and lateral projections. In fact, the duodenum may be later displaced or obscured by contrast-filled jejunal loops or by the stomach so that it is important not to overfill the proximal gut initially.
Once it has been established that the duodenojejunal junction is normally positioned, manual epigastric compression can help in the differential diagnosis between malrotation and normal duodenal motility [22]. Sometimes, an immediate contrast enema or delayed abdominal films may be necessary to identify the correct position of the cecum. While the position of the colon may be normal in a child with malrotation, 80 % of individuals with malrotation have an abnormal cecal position (Fig. 2.6). Generally, the shorter the distance between the duodenojejunal junction and the cecal apex, the shorter the length of the SB mesentery and the greater the risk of volvulus. Finally, an inversion of the normal relationship of the SMA and vein seen on US or CT has been described as suggestive of malrotation. Unfortunately, however, this is neither highly specific nor highly sensitive, but when noted should warrant further evaluation for malrotation with an upper GI exam [23].
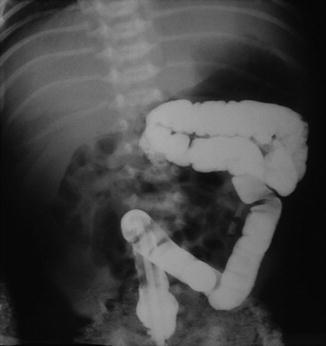

Fig. 2.6
Nonrotation of the colon. Radiograph shows that the entire colon is on the left
Barium Imaging of the Small Bowel in Crohn’s Disease
Crohn’s disease (CD) is a lifelong disease characterized by patchy, transmural inflammation, which may affect any part of the GI tract. The highest annual incidence of CD is 12.7 per 100,000 person-years in Europe, 5.0 person-years in Asia and the Middle East, and 20.2 per 100,000 person-years in North America [24]. The highest prevalence values for inflammatory bowel disease (IBD) are reported in Europe (ulcerative colitis [UC], 505 per 100,000 persons; CD, 322 per 100,000 persons) and North America (UC, 249 per 100,000 persons; CD, 319 per 100,000 persons). In time-trend analyses, 75 % of CD studies and 60 % of UC studies had a statistically significant increased incidence over time (P < 0.05) [24].
Establishing the diagnosis and exact distribution of the disease pattern with available local resources is crucial for planning the treatment strategy. A single gold standard for the diagnosis of CD is not available [25, 26]. For suspected CD, ileocolonoscopy and biopsies of both the terminal ileum and each colonic segment are the first-line procedures to establish the diagnosis, for they can verify microscopic evidence of CD. Irrespective of the findings at ileocolonoscopy, further investigation is recommended to examine the location and extent of the disease [25]. CD may affect a part of the ileum not reachable by the endoscope or may involve more of the proximal SB (10 % of patients). Additionally, at the time of diagnosis 15.5–36 % of patients have stricturing or penetrating disease (fistulas, phlegmons, or abscesses) [27, 28]. Endoscopy and radiology are complementary techniques to define the site and extent of the disease, which is important for the treatment plan [25].
Barium contrast examinations have long been the only imaging method providing morphological information of the SB, and have proven valuable in the diagnosis and management of CD. In the last decade, a progressive improvement of cross-sectional imaging has significantly changed the diagnostic and therapeutic work-up of patients [8, 29]. Indeed, these tools can reveal mucosal alterations as well as transmural and perienteric inflammation, leading to a new disease staging, detecting asymptomatic disease and assessing response to therapy [30]. For these reasons, on several systematic reviews and guidelines, modern cross-sectional imaging has replaced the barium studies for visualization of the SB in adult and pediatric populations [25, 26, 31]. In fact, there are definite advantages for the patient using the newer cross-sectional imaging modalities, particularly MR imaging, with the lack of ionizing radiation and increased accuracy in the detection of disease. There is, however, limited access to MR in most hospitals and the use of US depends on local expertise. For these and other reasons, to include cost, and the fact that they are noninvasive and easy to read and to perform, barium examinations are still the most available methods for investigating the SB in the majority of radiology departments [32]. Barium studies are a well-established method to investigate the SB in suspected or recurrent CD. In particular, SBFT remains the most commonly performed method for the investigation of SB diseases because of its ease of performance. SBFT can effectively depict transmural CD, but it may be imprecise in cases of mild disease, such as aphthous ulcers or other subtle mucosal abnormalities (Fig. 2.7). This disadvantage can be avoided with the use of enteroclysis, considered in the literature as the most accurate radiologic method in the diagnosis of CD [33]. In fact, it allows a careful estimate of disease extent, the identification of discrete lesions, characterization of strictures, and measurement of the length of unaffected bowel. Moreover, enteroclysis can depict early mucosal alterations, such as lymphoid nodular hyperplasia, aphthous ulcers, and valvulae conniventes swelling, particularly when double-contrast enteroclysis is performed with air instead of methylcellulose (Fig. 2.8) [34]. Finally, enteroclysis may help in the differential diagnosis between CD and granulomatous enteritis as well as ulcerative colitis. A newer application of barium studies is to exclude an SB stricture in patients with suspicious or known CD before performing CE.