(1)
Department of Vascular Surgery, Evangelisches Krankenhaus Königin Elisabeth Herzberge, Berlin, Germany
In this book all direct anastomoses between an artery and a vein are called AV fistulas even if the vein has been transposed from its original position either as part of the first operation or at a later stage (e.g., transposed brachiobasilic fistula).
3.1 Basics
3.1.1 Types of Arteriovenous Anastomoses
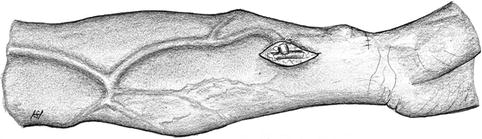
Basically there are three types of arteriovenous anastomoses (Fig. 3.1):
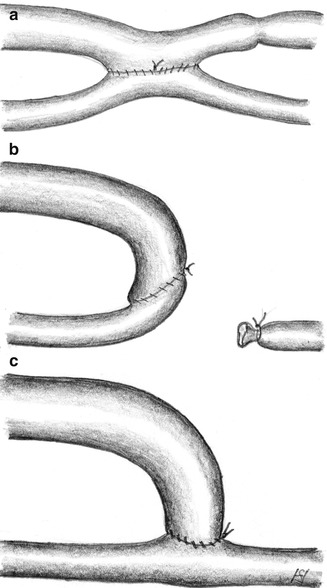

Fig. 3.1
Possible variants of AV anastomoses: (a) laterolateral, (b) terminoterminal, (c) lateroterminal
Laterolateral
Terminoterminal
Lateroterminal
A laterolateral anastomosis (Fig. 3.1a) lets blood flow in both directions. With functioning venous valves there is no distally-directed retrograde venous flow. Then the situation is the same as with a lateroterminal anastomosis. Insufficient valves, however, allow for the retrograde arterialization of the venous tree with subsequent venous stasis, edema of the respective extremity, and trophic disturbances. Therefore we disapprove of this type of anastomosis.
A terminoterminal anastomosis (Fig. 3.1b) requires the distal ligation of the artery. Thus an occlusion of the AV-anastomosis simultaneously also results in the proximal occlusion of the artery. Therefore we disapprove of this type of anastomosis, too.
With a lateroterminal anastomosis (Fig. 3.1c) the patency of the vein is preserved even if the feeding artery becomes occluded.
A lateroterminal anastomosis is the only reasonable option for AV fistulas.
Anastomotic Angle
Most frequently arteriovenous anastomoses are placed in the distal forearm or near the cubital fossa. In both positions we prefer perpendicular anastomoses. Near the wrist the anastomosed vein is fed both from the proximal and the distal arterial segments due to its low outflow resistance. That is why a right angle is advantageous. Similar flow conditions may prevail in the cubital fossa.
Diameter of the Anastomosis
Despite commonly held views, even an anastomotic diameter of more than 3–4 mm has almost no influence on the flow in the AV fistula. The flow rate is mainly determined by the arterial inflow resistance and the venous outflow resistance.
3.1.2 Pathophysiology of the AV Fistula
The palpable thrill in the anastomotic region is caused by pressure changes. The arterial inflow into the vein leads to:
Increased pressure
Pulsating thrill
Increased shear near the wall
Oscillations (changes of flow direction)
Morphologic alterations result in the dilation of the vein and frequently also in its elongation.
The extent of these changes depends on:
Hemodynamic conditions
The site of the arterialized vein
Other individual factors
The dilation of the vein decreases its outflow resistance. Consequently its flow increases. Furthermore the feeding artery undergoes changes. The increased outflow into the AV anastomosis raises its wall shear. The physiological adaptation to this stress also leads to the dilation of the artery. The resulting diminished arterial resistance further increases the flow rate in the fistula.
Owing to the manifold interdependent factors which influence changes of both artery and vein, different final states may occur (Fig. 3.2).
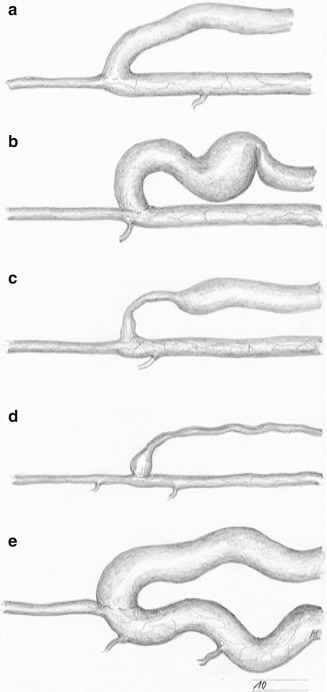

Fig. 3.2
Potential development of an AV fistula: (a) optimal outcome (even dilation of the vein), (b) dilation of the vein with kinking and aneurysm formation, (c) venous stenosis close to the anastomosis, (d) long high grade venous stenosis, (e) extreme dilation of the proximal artery
Ideally the arterialized vein becomes a large-diameter vessel with a sufficient flow, which may be punctured easily (Fig. 3.2a).
Quite often, elongation leads to stenoses caused by kinking (Fig. 3.2b). Upstream from there, aneurysmatic dilatations of the vein may develop due to the increased intraluminal pressure. Stenoses near the anastomosis frequently cause thrombosis in the postoperative weeks and months (Fig. 3.2c). Intraoperative tissue trauma and the particular mechanical strain of the curved path of the vein near the anastomosis are the most likely causes for these stenoses. General sclerosis of the entire arterialized vein, which leads to stenoses and occlusions, is relatively rare (Fig. 3.2d). This outcome is mainly observed with previously damaged veins (e.g., after chemotherapy, intravenous drug abuse). Likewise, an extreme dilatation of the feeding artery is relatively rare (Fig. 3.2e). Puncturing a fistula leads to alterations of the vein. These are described in detail in Chap. 17.
3.1.3 Special Surgical Aspects of AV Fistulas
Paying attention to numerous technical details when creating an AV fistula determines its early and late success rate.
Spasm of the Vein
Mainly in children and adolescents, the vein may become spastic after surgical exposure and dissection. This spasm can effectively be overcome by hydraulic dilatation using heparin solution. For this purpose a blunt canula is inserted into the vein, which is then gently dilated to its maximum diameter. The surgeon uses his gloved fingers to seal the canula and to empty the syringe. The assistant percutaneously compresses the vein. Thus the entire length of the vein (e.g., cephalic vein of forearm or upper arm) can be dilated step by step (Fig. 3.3).
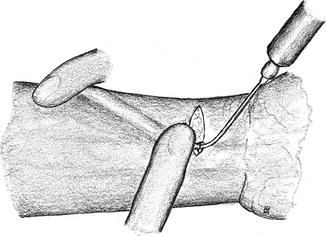

Fig. 3.3
Hydraulic dilation of the fistula’s vein
Spasm of the Feeding Artery
The artery may also become spastic during surgical exposure, especially in young patients. Anastomosing a spastic artery may lead to a narrow anastomosis with a diminished flow and subsequent thrombosis. Gentle spreading with a microdissector may solve this problem (Fig. 3.4).
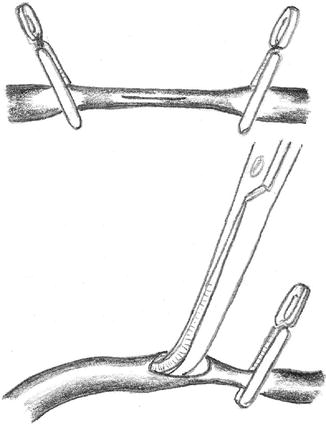

Fig. 3.4
Mechanical dilation of a small caliber artery using a microdissector
Rotational Error of the Vein
A venous torsion of only 30° may cause a high grade stenosis or occlusion (Fig. 3.5). Redoing the anastomosis is the only way to repair this situation. This technical mistake can be avoided if the correct rotation of the tightly-filled vein is marked (e.g., using a vascular clamp at its entry into the subcutaneous tissue) before creating the anastomosis (Fig. 3.6).
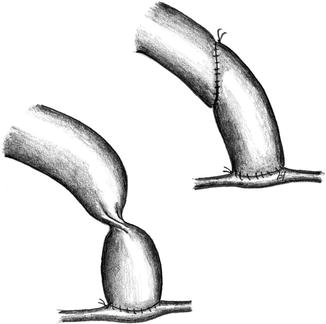
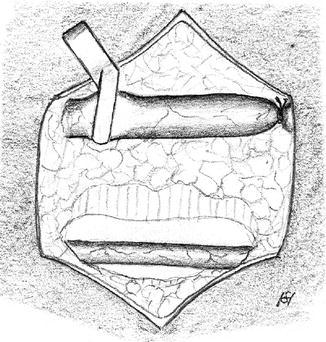

Fig. 3.5
Repair of a venous torsion which causes a stenosis by suturing a new anastomosis

Fig. 3.6
Surgical site after isolation of the cephalic vein and radial artery. To avoid torsion of the vein, its position should be marked by a standardized position of the clamp
Atraumatic Suturing Technique
Every trauma to a vessel increases the risk of thrombosis. The following guidelines for atraumatic surgery should be observed:
Grasp the adventitia only (Fig. 3.7)
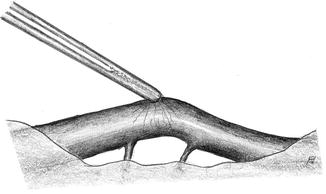
Fig. 3.7
Grasp only the adventitia for atraumatic handling of a vessel
Lift the vessel carefully using a dissector (Fig. 3.8)
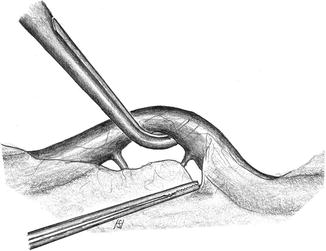
Fig. 3.8
Atraumatic manipulation of a vessel using a dissector
Use a fine pointed blade to open the clamped, tightly-filled artery (Fig. 3.9)
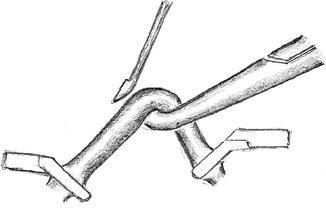
Fig. 3.9
Arteriotomy of a small artery. It is important to lift the tightly-filled artery to avoid injury to the posterior vessel wall
Use closed forceps gently to keep the vascular aperture open (Fig. 3.10)
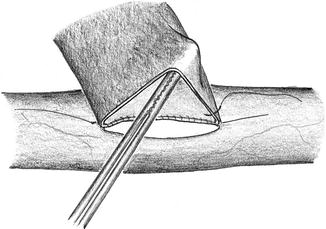
Fig. 3.10
Keep the vein open with closed forceps
Begin suturing at the less accessible side (Fig. 3.11)
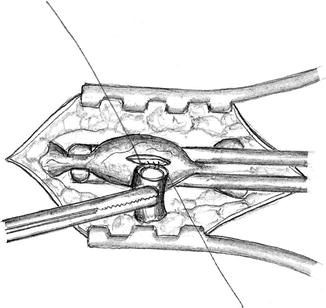
Fig. 3.11
Begin the suture at the most inaccessible side
Kinking of the Anastomosed Vein
Near the anastomosis, a curved path of the vein which avoids sharp turns is important. After declamping, the path of the vein has to be verified (lid retractor). If there is kinking, the strangulating subcutaneous tissue has to be removed (Fig. 3.12) unless the kinking is due to excess length (Fig. 3.13) of the vein. Then the vein should be shortened.
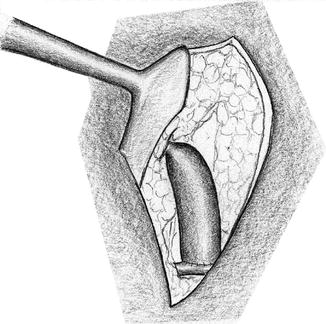
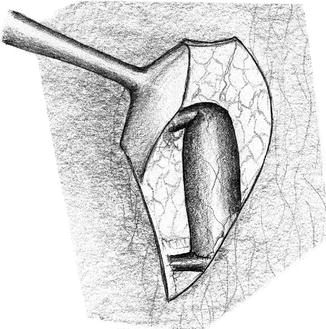

Fig. 3.12
Kinking of the vein due to strangulation of surrounding tissue

Fig. 3.13
Kinking of a vein which is too long
Intraoperative Prevention of Thrombosis
We flush the artery and the vein with heparinized saline before clamping.
3.2 AV Fistula of the Forearm
There are usually two or rarely three veins of the forearm which are suited for the creation of AV fistulas. Most often the cephalic vein can be used, and less frequently the basilic and median antebrachial veins.
3.2.1 AV Fistulas of the Cephalic Vein
Depending on an indivual’s cephalic vein, its arterialization may be undertaken at different levels.
Snuff Box Fistula
Indication/prerequisites
First access if suitable cephalic vein and strong pulse of the radial artery in the anatomical snuff box.
Contraindication
Expressed sclerosis of the radial artery in the forearm, as the flow will then remain insufficient.
Approach
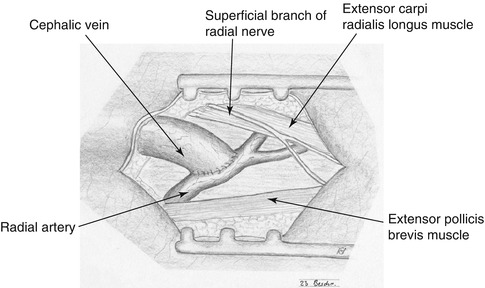
Longitudinal incision in the snuff box between the tendons of the extensor pollicis and abductor pollicis longus muscles (Fig. 3.14).
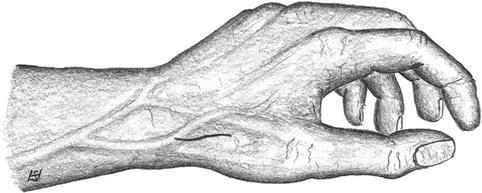
Fig. 3.14
Incision for snuff box fistula
Watch out for the superficial branch of the radial nerve during the exposure of the cephalic vein in the subcutaneous tissue.
Technical note
Micro instruments recommended.
Prognosis
From our own experience, around 15 % of patients develop inflammatory venous alterations with thrombosis in the motion segment of the wrist within the first year.
Evaluation/particularities
This is the most distal AV fistula. It increases the length of the venous segment that is available for puncture by around 4 cm as compared to the distal radiocephalic fistula. If thrombosis occurs, the dilated cephalic vein and the radial artery will usually still be well-suited for the creation of a distal forearm fistula.
Distal Radiocephalic Fistula of the Forearm
Indication/prerequisites
Well-developed cephalic vein up to the forearm and strong pulse of the radial artery at the wrist.
Contraindication
Expressed sclerosis of the radial artery in the forearm, as then the flow is and will remain insufficient.
Approach
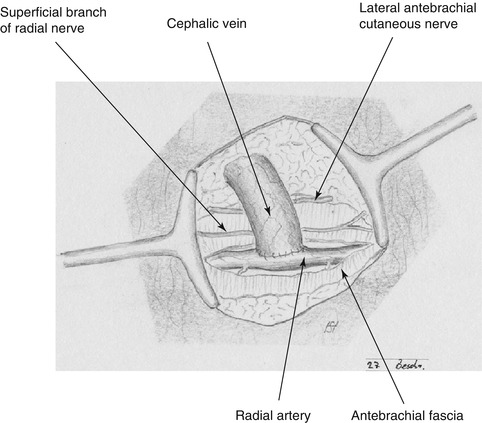
The choice of incision depends on the distance between artery and vein. If they lie close to each other, we suggest a 2–3 cm transverse incision (Fig. 3.17). If there is a longer distance between the vessels, two separate longitudinal incisions are recommended (Fig. 3.18).
Fig. 3.17
Incision for distal cephalic fistula if radial artery and cephalic vein are close to each other (radiovolar transverse incision)
Fig. 3.18
Incisions for distal cephalic fistula if radial artery and cephalic vein are not close to each other (two longitudinal incisions)
Sufficient exposure of a venous segment while preserving the radial cutaneous antebrachial nerve and the superficial branch of the radial nerve.
Longitudinal incision of the antebrachial fascia and exposure of the radial artery medial to the flexor carpi radialis muscle.
Technical note
Micro instruments are recommended for small size vessels.
Prognosis
This is the AV fistula with the best long term function. Under favorable circumstances it may be used for more than 25 years.
Evaluation/particularities
Most important native AV vascular access. To be preferred as first access.
Proximal Cephalic Fistula
Indication/prerequisites
Frequent secondary procedure after distal cephalic fistula has failed. Possible with unsuitable distal segment of the vein but suitable cephalic vein in the proximal forearm.
Approach
Longitudinal incision down to the fascia at the medial margin of the brachioradial muscle in the proximal forearm.
Exposure of the vein from a separate longitudinal incision. (Please note that the artery lies relatively far beneath the surface. Thus the distance between artery and vein may also be quite long.)
Longitudinal incision of the antebrachial fascia at the medial margin of the brachioradial muscle while preserving the superficial branch of the radial nerve.
Distal ligation of the vein and shifting of its proximal stump to the artery.
Trimming of the vein for correct length.
Prognosis
Good long term results.
Evaluation/particularities
Decisively shorter segment for puncture at the forearm, but making good use of still suitable vein segments there.
3.2.2 AV Fistulas of the Basilic Vein
Distal Basilic Fistula
Indication/prerequisites
Unsuitable cephalic vein in the forearm but suitable basilic vein and ulnar artery in the forearm.
Approach
Close to the wrist the distance between the basilic vein and the ulnar artery tends to be relatively long. Therefore two separate longitudinal incisions are made.
Isolation of the basilic vein before deciding on a more proximal incision for the ulnar artery.
Exposure of the ulnar artery medial to the tendon of the flexor carpi ulnaris muscle while taking care not to injure the ulnar nerve (Fig. 3.23).
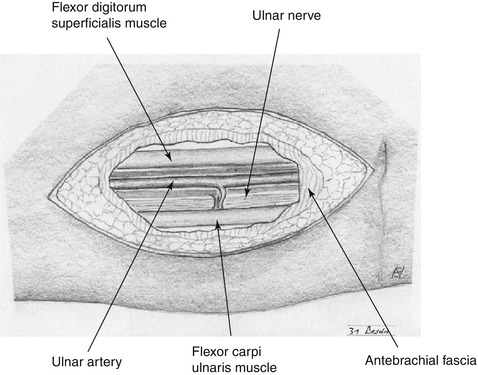
Fig. 3.23
Anatomy of ulnar artery in the distal forearm
Pulling the proximal end of the distally ligated basilic vein through a subcuticular tunnel to the ulnar artery while preserving the integrity of the dorsalis manus branch of the ulnar nerve.
Trimming of the vein for correct length.
Prognosis
Good long term results despite expressed tendency of the basilic vein to dilate more than desired.
Evaluation/particularities
Frequently neglected access due to “rolling” veins, difficult puncturability, and difficult positioning of the arm during dialysis. The fistula is punctured while flexing the elbow. It is easier when the skin is stretched. Correct positioning of the arm is facilitated by using cushions or folded towels.
Proximal Basilic Fistula
Indication/prerequisites
Unsuitable cephalic vein but suitable basilic vein and suitable ulnar artery in the forearm.
Same procedure as with the distal basilic fistula. It is important to note the considerably deeper and more medial position of the ulnar artery between the two heads of the flexor carpi ulnaris muscle. In rare cases the radial artery can be used to feed the basilic vein (Fig. 3.26). This may be realized in the case of a small diameter ulnar artery and a large diameter radial artery (e.g., after a previous cephalic fistula). However, the longer distance to the radial artery is a disadvantage.
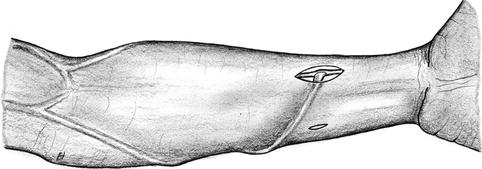

Fig. 3.26
Arterialization of the basilic vein via the radial artery
Prognosis and evaluation
Similar to distal basilic fistula, but definitely shorter venous segment that can be punctured.
Transposition of Basilic Vein in the Forearm (Fig. 3.27)
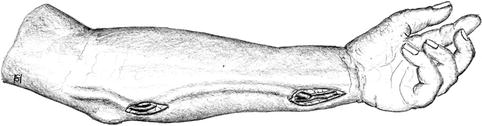

Fig. 3.27
Superficialization of the arterialized basilic vein in the forearm
In our opinion the superficialization of a brachiobasilic fistula in the forearm should preferably be delayed so as first to wait and see how it matures.
Indication
Fixation of an extremely mobile, and/or deeply-imbedded, and well-dilated basilic vein to facilitate puncturing.
Approach
Isolation of the arterial anastomosis and of the basilic vein near the cubital fossa beginning with volar longitudinal incisions.
Ulnar incisions to free the entire vein in the forearm.
Dissection of the vein near the anastomosis, and removal of the vein from its natural bed.
Creation of a volar, subcutaneous tunnel, passage of the vein, and reestablishment of the vascular continuity.
Prognosis and evaluation
Very rare indication. Good long term results.
3.2.3 AV Fistula of the Median Antebrachial Vein (Fig. 3.28)
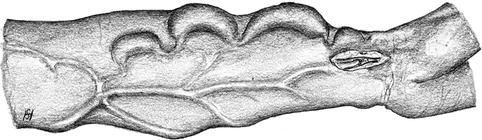
Fig. 3.28
Arterialization of the antebrachial vein
Indication/prerequisites
Unsuitable cephalic and basilic veins in the forearm but suitable median antebrachial vein.
Approach
The median antebrachial vein should be anastomosed either to the ulnar or to the radial artery, as due to its anatomical varieties either one might be better suited than the other. Depending on the anatomy, one or two longitudinal incisions are necessary.
Prognosis
Limited experience. Under particular circumstances this access may serve for several years but tends to develop scarred stenoses.
Prognosis and evaluation
Rare indication mostly after previous forearm AV fistulas.
3.2.4 Repair of AV Fistulas in the Forearm
In the long run, various (morphologic) changes occur that impair the function of a vascular access. These encompass:
Thromboses
Venous stenoses due to tissue proliferation or kinking
Venous aneurysms
Runoff mainly through side branches
Difficult puncture due to deep path of the vein
Retrograde venous perfusion of the forearm due to central venous stenosis or occlusion
Reduced arterial perfusion of the hand
Infections
Stenoses of the feeding artery
Frequently several mutually influencing pathologic changes coexist. When correcting these changes, care should be taken to preserve the venous segments suitable for puncture.
Thrombosis of Forearm AV Fistulas
Any operation should:
Find the underlying cause of a thrombosis.
Remedy the cause.
Restore the previous function (thrombectomy).
A thrombectomy is a demanding procedure which requires extensive experience. Based on the presentation and the pathogenesis of a thrombosis, we distinguish between three forms: perioperative thrombosis, early thrombosis, and late thrombosis.
Perioperative Thrombosis
Definition
Thrombosis within the first 48 h after surgery.
Causes
Morphologic alterations of the vein.
Stenoses of the artery, the anastomosis, or the vein possibly caused by surgical mistakes or compression (hematoma).
Hypercoagulability.
Altered vessels (traumatic surgical technique, previous chemotherapy, IV drug abuse).
External compression (dressing).
Therapy
The wound should be reopened as soon as possible to correct potential morphologic causes. If a stenosis results from a rotational error, kinking, or an anastomosis which is simply too narrow, a new anastomosis should be created after thrombectomy. If these causes can be excluded, a transverse venotomy close to the anastomosis is followed by thrombectomy and examination of the thrombi.
Should mainly white thrombi prevail, most likely hypercoagulability exists. Then laboratory tests for coagulation disorders should be ordered. After a successful thrombectomy, temporary systemic heparinization is subsequently switched to oral anticoagulation.
Should red thrombi prevail, an increased thrombogenicity of the vessels is likely. Then the postoperative administration of heparin for 3–5 days should be sufficient for the fistula to remain patent. Should chronic vascular damage have been caused by previous injections (chemotherapy, IV drug abuse), continuous use of anticoagulants is recommended.
Early Thrombosis
Definition
Thrombosis occurring between 2 days and 2 weeks after surgery.
Causes
Early thrombosis is frequently observed with previously damaged veins (chemotherapy, IV drug abuse). Sometimes the vein can be palpated as a thin and fibrous strand.
Therapy
Thrombectomy should always be attempted. If no morphologic cause close to the anastomosis is suspected, we recommend approaching the vein proximal to the previous incision (for the anastomosis) because of almost certain scarring.
Late Thrombosis
Definition
Thrombosis after the second week post surgery.
Causes
History taking and clinical examination mostly suffice to discover the probable cause. Stenoses of the anastomoses, stenoses of the vein, aneurysm, and infections are likely. Likewise, an abacterial inflammation of the venous segment close to the anastomosis is a possible cause. Erythema and an increased sensitivity to touch can frequently be found.
Therapy
Thrombectomy is performed via an incision proximal to the distal venous segment. If the suspected morphologic cause is situated close to the anastomosis, the site of the skin incision should be selected based on the distance between artery and vein in view of a potential proximalization of the anastomosis.
Approach for a short distance between artery and vein
The vein is exposed from a transverse incision proximal to the anastomosis followed by thrombectomy (Figs. 3.29 and 3.30). If this thrombectomy maneuver is futile, the transverse incision is extended volarly towards the artery so as to create a new proximal anastomosis (Fig. 3.31).
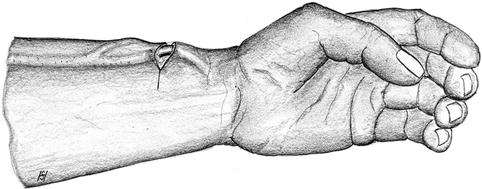
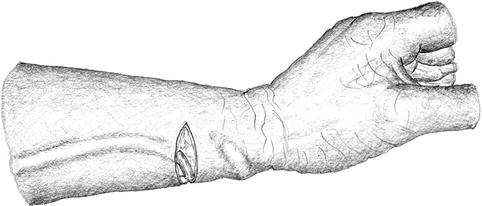

Fig. 3.29
Incision for thrombectomy of distal cephalic fistula (short distance between artery and vein)

Fig. 3.31
Extension of the transverse incision and proximalization of the anastomosis if thrombectomy fails (cf. Fig. 3.29)
Approach for a long distance between artery and vein
The vein is exposed via a longitudinal incision proximal to the anastomosis (Fig. 3.32).
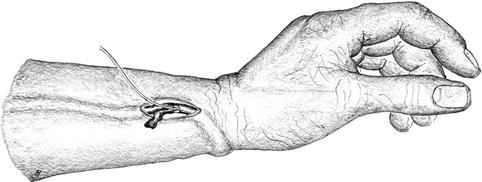

Fig. 3.32
Incision (longitudinal) for thrombectomy of distal cephalic fistula with far distance between artery and vein
If an attempt at thrombectomy is futile, the artery is exposed via an additional longitudinal incision. Depending on the distance between artery and vein and the state of the remaining vein, either the shortened vein is reanastomosed to the proximal artery (Fig. 3.33), or a graft is implanted to re-establish vascular continuity (Fig. 3.34).
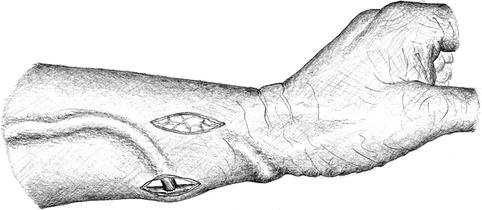
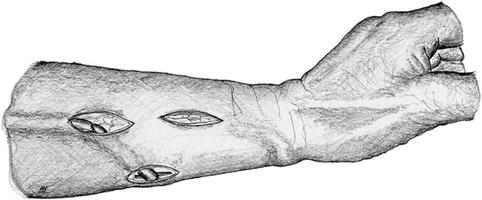

Fig. 3.33
Isolation of the vein and creation of a new proximal anastomosis (two incisions) if thrombectomy (one incision) fails (cf. Fig. 3.32)

Fig. 3.34
Arterial feeding of the vein via a short interposition graft if thrombectomy fails (cf. Fig. 3.33)
Venous Stenosis of Forearm AV Fistula
Possible causes are intraluminal tissue proliferation resulting from hemodynamic changes, inflammatory shrinking of the lumen (frequently after chemotherapy or IV drug abuse), or kinking (Fig. 3.35). The latter goes together with the (desired) dilation of the vein.
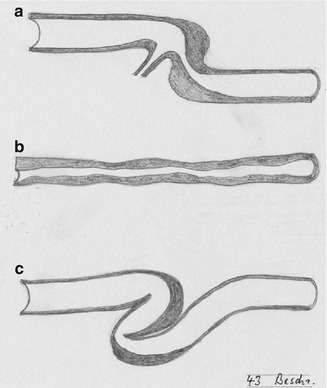

Fig. 3.35
Possible venous stenoses: (a) close to a side branch, (b) long “inflammatory” stenosis, (c) kinking
Diagnosis
To palpate a superficially-running vein over its entire length is frequently sufficient for its evaluation. This proves more difficult if the vein runs further beneath the surface. In addition to routine color-coded duplex sonography we advocate phlebography before complex reconstructive surgery. Placing the needle into the vein also allows for retrograde imaging of the artery.
Therapy
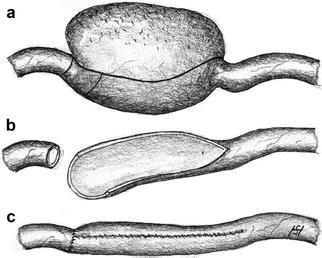
Unless caused by kinking, short stenoses should primarily be treated by percutaneous transluminal angioplasty (PTA). Recurrent restenoses or stenoses refractory to PTA are amenable to surgery. Both patch plasty and interposition grafts are feasible options (Fig. 3.36). Stenoses due to kinking usually require surgery. It is almost always possible to resect the elongated, kinking segment and to create an end-to-end anastomosis (Fig. 3.37). A long highly-stenosed or occluded segment of the vein, however, should be replaced by a graft for future punctures (Fig. 3.38). It is also possible to mobilize and transpose the distal stump of a proximally occluded cephalic vein to a suitable side branch which drains into the basilic vein in the forearm so as to preserve puncturability (Fig. 3.39). Likewise, a distal brachiocephalic fistula which is occluded proximal to the cubital fossa can be preserved if it is anastomosed to the basilic vein there (Fig. 3.40).
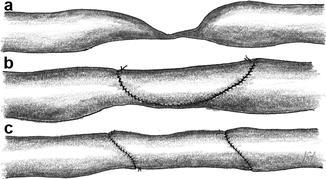
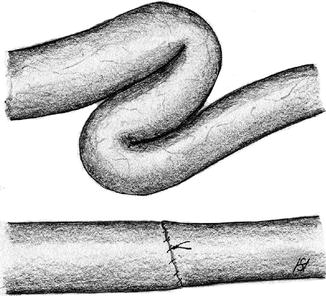
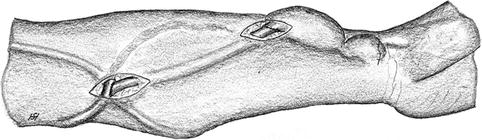
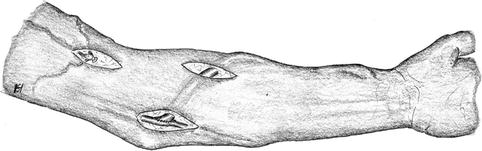

Fig. 3.36
Repair of a venous stenosis (a) using a patch (b) or a graft (c)

Fig. 3.37
Resection of a kinking venous segment

Fig. 3.38
Using a prosthetic bypass (diameter of at least 7 mm recommended) to circumvent a long stenosis of a distal cephalic fistula

Fig. 3.39
Proximal occlusion of a distal radiocephalic fistula. Creation of a new runoff via a side branch to the basilic vein

Fig. 3.40
Proximal venous occlusion of a distal cephalic fistula. Salvage of the fistula by transposing the cephalic vein into the basilic vein
Aneurysms
Aneurysms occur because the venous walls are rather thin. Nearly all aneurysms develop at frequent puncture sites with the already frail scar tissue of the venous wall being submitted to repetitive extra strain. Furthermore, the intraluminal pressure can also be too elevated for the thin vessel wall as the result of a high arterial influx or a venous outflow that is impaired by stenoses (Figs. 3.41 and 3.42).
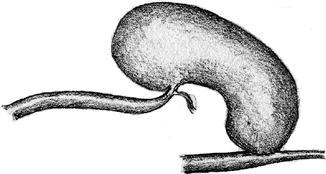
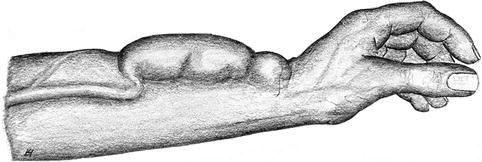

Fig. 3.41
Kinking of the arterialized vein caused by an upstream aneurysm

Fig. 3.42
Aneurysm of a distal radiocephalic fistula
Diagnostics
Morphologic and clinical findings (inspection and palpation) as well as other (compounding) factors influence the indication for surgery. Duplex sonography and angiography via venipuncture with retrograde arterial imaging can add extra useful information preoperatively. Diagnostic interests concern:
The morphology of the vein including its central runoff
Flow volume
Indications for surgery
Important criteria are:
Aneurysm size
Risk of rupture
(Partial) thrombosis
Functional restrictions
Combination with stenosis
Cosmetics
These morphologic and functional aspects have to be weighed against:
Risk for septic complications when using prosthetic grafts
Complexity of the operation
Life expectancy of the patient
Feasibilty of future AV access surgery
Therapy
There are basically four different surgical options:
1.
Resection of the aneurysm with direct end-to-end anastomosis.
2.
Partial resection of the aneurysm wall and closure with (running) suture either leaving the vein in situ or creating a new extra tunnel for the vein.
3.
Resection of the aneurysm and interposition of an alloplastic graft.
4.
Creation of a new AV access with (delayed) ligation of the pre-existing aneurysmatic vascular access.
Resection of the Aneurysm with Direct end-to-end Anastomosis
Indication/prerequisites
Short aneurysm, end-to-end anastomosis feasible.
Contraindication
(Local skin conditions).
Approach
Longitudinal incision over the aneurysm.
Technical notes
Appropriate incision to resect the superfluous skin (Fig. 3.43) if applicable.
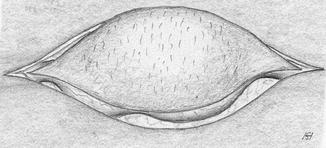
Fig. 3.43
Elliptical skin excision over the aneurysm
Exposure of the aneurysm.
Exposure of the afferent and efferent venous segments.
Resection of the aneurysm with end-to-end anastomosis (Fig. 3.44).
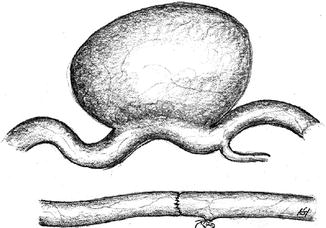
Fig. 3.44
Repair of an aneurysm via excision and direct suture
Prognosis
Good long term results.
Distinctive feature
The fistula runs directly through the scarred area.
Partial Resection of the Aneurysm Wall and Closure with Direct Suture
Indication/prerequisites
AV access worth preserving.
Contraindications
Extremely thin skin.
Lack of subcutaneous cover.
Approach
Longitudinal incision over the aneurysm.
Technical notes
Appropriate skin incision in order to simultaneously resect the superfluous skin.
Exposure of the aneurysm and of potential stenoses (Fig. 3.45).
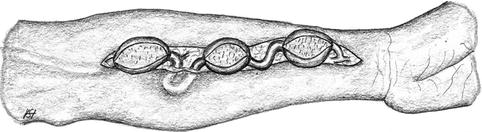
Fig. 3.45
Long circular exposure of the aneurysmatically-altered vein
Resection of the stenosed segment and partial longitudinal resection of the vessel wall leaving behind enough tissue to create a new lumen (Fig. 3.46).